|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Langmuir I. — Phenomena, Atoms and Molecules |
|
|
 |
| Предметный указатель |
Interfacial surface tension measurements 73
Intermolecular forces 267
Interphase surface mobility 370
Inverse 9th power law 266
Inverse square law 262
Inverse tenth power law 219 263
Iodide 82 83
Iodide ions 255
Iodine 49 82
Iodine atoms 255
Iodine-COO 82
Ion current 361 362
Ion emissions 323 327 358
Ion evaporation 345 351 354 355
Ion evaporation rates 353 354 363
Ion saturation current 363
Ionic 221
Ionic charges 263
Ionic molecules 260
Ionizing potential 53 54 58 345
Ions 5 53 54 55 56 57 59 60 61 72 148 201 204 219 220 222 225 227 229 232 256 260 261 262 263 286 322 323 324 327 328 336 351 352 357 359 366 386 388 393 397 399
Iron 121 134 142 144 397
Iso- 83
Iso-compounds 83 283
Isoelectric 243 256
Isoelectric isosteres 245
Isolated active spots 341 344
Isomorphism 247 248 249 250 251 253 256
Isomorphous substances 247
Isosteres 242 243 245 246 256 257
Isosteric 244
Isosteric compounds 242
Isosterism 243 248 257
Isosterism of comolecules 245
Isotherm 59
Isotope separation 26
Japan 30 31
Jeans, J.H. 380
Johns and Langmuir tables 336
Jour. Amer. Chem. 146 241 294 300
Jour. Amer. Chem. Soc. 83 94 112 114 124 126 140 193 197 205 207 255 259 298 301 302 305 317 323 399
Jour. Amer. Electrochem. Soc. 324
Jour. Frank. Inst. 294
Jour. Ind. Eng. Chem. 205
Jour. Phys. Chem. 29 97 284
Journal of Physical Chemistry 75 270
K 40 57 71 115 129 135 166 173 179 181 182 183 186 188 189 192 198 200 203
K, q 187 225
Kelvin scale 112
Kernel 395 396 397 398 399
Kernel charge 396
Kernel of an atom 391
Kilgore Bill 23
Killian 353 356
kinetic energy 46 52 310 316
Kinetic theory 40 41 61 120 128 189 204 218 219 259 263 296 298 326
Kinetic theory of gases 2 60 258 380
Kingdon, K.H. 140 323 349 350 351 352
Kingdon, K.H., Phys. Rev. 349
Knudsen 163 164 165 169 207 297 299 306 307
Knudsen’s accomodation coefficient 307
Konowalow’s 84
Kopp 247
Koref 317
Kossel 260
Kremlin 19
Krypton 266
Kundt 163 202 298 299
Labor unions 16
Lambert’s cosine law 326
Lamps 101 102 104 105 106 107 108 132 152 153
Lande, A. 140 219 263
Landolt — Boernstein tables 230 241
Langmuir 94 112 114 124 126 146 193 195 196 260 293 296 301 323 343 349 350 351 352
Langmuir and Orange 152
Langmuir, Gen. Elec Rev. 144
Langmuir, I., Chem. Met. Eng. 139
Langmuir, I., Jour. Chem. Phys. 354
Langmuir, I., MacLane and Blodgett, K.B., Phys. Rev. 337
Langmuir, I., Proc. Nat. Acad. Sci. 373
Langmuir, Ibid 128
Langmuir, Jour. Amer. Chem. Soc. 113 128 130 132 135 139 150 160 205 284 325 370 377 386 388 396
Langmuir, Jour. Frank. Ins. 241
Langmuir, Phys. Rev. 207 216 293 324 349 381
Langmuir, Proc. Amer. Inst. Elec. Eng. 131
Latent heat of evaporation 40 226 237 238 315
Latimer 400
Lattice constant 325
Lattices 67
Laue 67
Law of excluded middle 7
Law of ideal gases 32
Law of survival of the fittest 10
Law of uniformity of nature 7
Laws of cause and effect 4
Laws of chance 5
Laws of elastic collision 312
Laws of heat conduction 162
Laws of heat convection 147
Laws of the kinetic theory of gases 33
Lead and silver salts 244
Lead cooling correction 337
Lend-lease 28
Leningrad 27 29
Leningrad and Moscow 17
Lewis & Randall’s “Thermodynamics” 135
Lewis and Randall 114 122
Lewis, G.N. 114 241 260 388 391 399
Light intensity 286 362
Lindemann 309
Liquid 39 41 42 67 267 397
Liquid air 63 64 289 290 291 292 295 297 301 337 385
Liquid hydrocarbon 233 267 271 275 280
Liquid or solid hydrogen 297
Liquid phase 76 87
Liquid phase to the vapor 79
Liquids in bulk 273
Logic 7
London 220
Long range forces 56
Loss by convection 106
Low carbon steel 145
Lyman 130
Lysenko, T.D. XI
M 59 118
Mackay, G.M.J. 114 124 146 293
Mackay, G.M.J., J. Franklin Inst. 117
Mackay, Jour. Amer. Chem. Soc. 128 160 205
Mackenzie King 24
Maginot Line 21
Magnanini 146
Magnesium 35 144
Magnesium carbonate 247 248 250
Magnesium ions 246 248
Magnesium oxide 121 142 143 246
Magnesium salts 253
magnetic susceptibility 242
Magnuson and Kilgore Bills 22
Magnuson Bill 23
Manganese 121 123 144
Manganese atoms 256
Manganese ion 255
Manhattan project 13
Massachusetts Institute of Technology VIII
Mathematical equation 3
Maxwell 2 163 258 305 314
Maxwell — Boltzmann distribution (M.B.D.) 371
Maxwell’s distribution 165 316
Mayer 61
McGraw-Hill 114 129
| McLeod gauge 33
Mean energy of molecules 307
Mean free path 163 170
Mean free path of electrons 287
Mean free path of the gas molecules 166
Mean free path of the molecules 33 301
Mean kinetic energy 306 310
Mean specific heat of hydrogen 194
Mean thermal velocity 314
Megabar 208
Melting 68
Mercury 49 69 70 112 297 317
Mercury atoms 295 297
Mercury vapor 149 232
Mercury vapor and nitrogen 106
Met. Chem. Eng. 259 284
Metal atoms 297
Metallic caesium 54 375
Metallic oxides 35 113
Metallic state 113
Metallic substances 398
Metallic thorium 38
Metaphosphates 250 251 253
Metastable atoms 285 286
methane 41 64 237 257
Methane molecules 260
Methanol 73 83 84 87
Methanol-Ethanol 81
Methyl 69
Methyl acetate-Ethyl acetate 81
Methyl acetatethyl acetate 81
Methyl groups 69 267
Meyer, O.E. 167
Meyer’s “Kinetische Theorie der Gase” 167
MgO 121
MgO, BN, 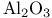 394 394
MICA 62
Migration 355 370 386
Migration of ions 234
Migration of the molecules 236
Millikan 299 300
Mitscherlich 247
Mitscherlich’s rule 244 246
Mixed crystals 253 347
Mixture Energies 81 83 84
Mn** 399
Mobility 46 47 68 327 385 399
Mobility and surface diffusion coefficient of adatoms 379
Mobility of adatoms 381
Modified Boltzmann equation 268
Mol 86
Mol-fraction 78 80 87 239
Molar Polarization 224
Molecular 120
Molecular dissymmetry 258
Molecular heat 165
Molecular hydrogen 35 114 116 117 123 133 137 141 147 151 160 161 193 198 201 204 209 210 212
Molecular nitrogen 300
Molecular or atomic weight 380
Molecular orientation 266 267
Molecular oxygen 135
Molecular refractive index 265
Molecular surface S 40
Molecular Volume 41 237
Molecular volume V 40
Molecular weight 34 69 83 165 224 269 270 273 297 397
Molecule is uncharged 264
Molecules 2 5 7 39 40 41 42 45 46 61 63 67 70 71 72 74 76 78 88 91 95 98 105 113 114 163 164 170 178 180 201 207 208 212 215 216 218 219 221 222 223 230 234 245 258 261 264 265 267 270 272 274 276 277 279 280 283 295 296 305 308 310 315 316 318 320 400
Molecules of gas 164
Molotov 24 30
Molybdenum 125 144 305 397
Molybdenum atom 305
Molybdenum carbonyl 397
Molybdenum filaments 301
Monatomic film 35 37 38 39 49 54 64 325 337
Monatomic gas 120
Monatomic layer 322 327
Monatomic oxygen 38
Mond, Hirtz, Cowap, J. Chem. Soc. 397
Mono-, di- and tribasic alcohols 77
Monobasic alcohols 238
Monochloro acids 230
Monoclinic mixed crystals 253
Monoclinic system 252
Monomolecular 272
Monomolecular film 50 231
Moon 366
Morality 11
Moratoriums on Mortgages 16
Moscow 19 28 30
Moscow News 19
Moser’s breath figures 293
Mott-Smith, Harold 232
Murphy, Walter J. 17
Mutations 6 7
Mutual solubilities of liquids 85
Myristic acid 44 282 283
n-Octyl alcohol 41
NaCI 225
NaCI,  , ,  394 394
NaCI, KI 219
National Academy of Sciences 23
National defense 22
Natural laws 4
Naziism 30
Nearly saturated films 373
Nearly saturated vapor 377
Negative by positive ions 255
Negative double refraction 244 249 252
Negative ions 223 392
Negative residual charge 396
Negative surface energy 84
Negative valence 388 390 393 395—398
Neon 245 261 285
Neon atom 245 260 286
Nernst 127 146 195 315
Nernst equation 71
Neutral atoms 390
Newton 228
Newton’s laws 1
Ni(CO) 397
Nickel salts 253
Nietzsche 9
Nitrate ion 253
Nitrates 246 250 251 253 254 257
Nitrates and Carbonates 249
Nitrogen 62 103 104 105 106 107 108 121 124 134 149 153 158 160 169 198 200 202 204 241 244 245 255 257 266 299 305 396
Nitrogen atoms 255
Nitrogen molecule 305
Nitrogen, argon, and mercury vapor 126
Nitrous oxide 241 242 244 245
NO 399
Non-Polar Gases 62
Non-polar liquid 226
Non-polar Molecules 61 72 221 223 224 227 228 260 261 262 264
Non-polar solvents 229
Nonane 237
Normal free path 301
Novosibersk 19
Nuclear physics 29
Nucleus 5 6 218 219 221 229 260 261 265 354 371 372 389
Nucleus formation 293
octane 41 42 97 282 283
Octet 41 253 399
Octet compounds 255
Octet equation 255 256
Octet theory 241 243 244 246 247 251 255 257 390
Octet theory of valence 249 256
Octopole 222
Octyl alcohol 41 42
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



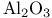
 ,
, 