Авторизация
Поиск по указателям
Rao C>N> — Ultra-Violet and Visible Spectroscopy: Chemical Applications
Обсудите книгу на научном форуме Нашли опечатку?
Название: Ultra-Violet and Visible Spectroscopy: Chemical ApplicationsАвтор: Rao C>N> Аннотация: It would be superfluous to stress the importance of electronic .spectroscopy in structural or analytic research. It has now become a matter of routine to record the ultra-violet or visible spectra of compounds for purposes of identification or structure elucidation. The spectrophotonletric methods of analysis have replaced the conventional methods in ever so rnany instances. The number of-publications in the different branches of chemistry, reporting data on or making use of electronic spectroscopy, is ever-increasing and it would be impossible to review the field completely. In this book, I have tried to introduce the basic concepts of electronic spectroscopy and to present its applications in analytical, structural and physico-chemical problems. As far as possible, the rnateripl has been selected to illustrate the basic: principles of modern theories in chemistry. While surveying the data on organic molecules, several empirical generalizations have been discussed. lVlany of the more recent developments like tIle far ultra-violet spectra of organic molecules, fluorescence spectra, charge transfer spectra, ligand field theory, optical rotatory dispersion etc., have been briefly discussed in the latter part of the book. All through the discussion, the recent notations of electronic transitions have been used, although the other commonly encountered notations have also been mentioned. I shall be most gratified if this book can be of help to chemists in using electronic spectroscopy more effectively in research or routine work.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: 1st editionГод издания: 1961Количество страниц: 164Добавлена в каталог: 30.08.2009Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
132 66 75 29 88 65 105 26 10 10 'Loose bolt' effect 102 'No bond' configuration 115 116 1,1'-Diethyl-2,2'-cyanine 136 1,2,3-Triazole 52 1,3-Dialkyl-5-imino-tetrazoles 59 1,3-Diphenylcyclobutadiene 49 1,4-Diarylbutadienes 106 1,6-Diphenylhexatriene 106 1-Alkenes 94 1-Methyldecapentaene- 101 1-Nitropropene 25 1-Octyne 94 1:1-Dicyclohexenyl 27 1:3-Butadiene 25 26 2,2'-Dibenzothiazolyl disulphide 108 2-Alkyl-1-alkenes 94 2-Ethylhex-1-en-3-one 29 2-Ethylhex-2-en-1-al 29 2-Hydroxypyridine 55 2-Mercaptobenzothiazole 72 2-Methyl-1-acetyl-cyclohexene 88 2-Methyl-5-alkylamino tetrazoles 59 2-Nitrobiphenyl 89 2-Octyne 94 2-Pyridone 56 2:3-Dimethylbutadiene 27 2:3:4:5-Tetrahydroacetophenone 29 2:4-Dinitrophenylhydrazones 30 75 2:4-Hexadiene 26 3-Hydroxypyridine 56 3:4-Dimethylpent-3-en-2-one 29 4,4'-Diaminobenzophenone 143 4,5-Dimethylchrysene 90 4,5-Dimethylphenanthrene 90 5-Nitro-2,4-pentadiene 25 5:6-Dimethylbenziminazol 63 7-Dehydrochlolestene 26 7-Dehydrocholesterol 26 8-Hydroxyquinoline 104 8-Hydroxyquinoline salts 104 9,9'-Phenanthroin 106 Abietic acid 26 66 Absorbance 3 Absorbancy, standard values 6 Absorption cells 7 Absorption frequencies, spread 117 Absorption intensity 3 Absorption spectra 115 Absorption spectra of alkali halides 112 Absorption spectra, far ultra-violet 92 Absorption spectra, low-temperature 108 Absorption spectra, presentation 4 Absorption, irrelevant 73 Acetaldehyde 17 101 Acetanilides 47 Acetone 11 17 19 23 101 143 Acetone photochemical, decomposition 101 Acetophenone 19 43 85 Acetophenones 47 Acetophenones, substituted 86 Acetylacetone 71 Acetylenes 94 Acetylenic compounds, naturally occurring 36 Acidity function 76 acids 100 Acids, 30 Acids, dissociation constants 75 Acraldehyde 29 Acridine 53 102 Acridine dyes 102 Acridone 102 Adenine 58 Alcohols 93 100 Aldehydes 75 Aldehydes, 28 Aldehydes, aliphatic 101 Aldehydes, asymmetric 138 Aliphatic aldehydes 101 Aliphatic compounds 101 Aliphatic ketones 101 Alkali azides 131 133 Alkali halides 104 131 Alkali halides, paramagnetism 132 Alkaline earth azides 131 133 Alkaline earth halides 104 Alkaloids 65 Alkenes 93 Alkyl nitrates 22 Alkyl nitrites 21 Alkyl substitution 17 Allenes 94 Alloxan 57 Amethyst violet 103 Amides 148 Amides, cyclic 96 Amine picrates 75 Amino acids, aromatic 148 Amino-1,2,3,4-thiatriazoles, 5-substituted 59 Amino-pyrimidines 57 Aminopyrene sulphonic acids 105 Angstrom unit 1 Aniline 41 42 44 Anilines 47 144 Anisoles 47 Anthocyanidins 135 Anthocyanins 135 anthracene 48 53 95 101 102 107 Anthraquinone 102 Arachidonic acid 78 Aromatic carbonium ions 134 Aromatic complexes with oxalyl chloride 117 Aromatic negative ions 134 Aromatic non-benzenoid 49 Aromatic positive ions 134 Aromatic substitution, orientation in 47 Aromatics 92 95 Aryl disulphides 108 Aryl sulphones 47 Aryl sulphoxides 47 Association constants 79 Association phenomenon 136 Auxochromes 9 10 15—17 Azine dyes 102 Azines, monocyclic 55 Azobenzene 43 69 134 Azoxy compounds 21 Azulene 141 Azulenes 49 Bands, 'Soret' band 135 Bands, A-bands 11 Bands, E-bands 11 Bands, F-bands 132 Bands, K-bands 11 25 27 132 Bands, M-bands 133 Bands, R-bands 11 17 22 133 Bands, Rydberg 92 Bands, V-bands 132 Barbituric acid 57 Bases, dissociation constants 75 Bathochromic shifts 12 Beer — Lambert law 3 Beer — Lambert law, validity 3 Benesi — Hildebrand method 119 Benzaldehyde 19 Benzaldehydes 47 benzene 11 12 45 53 95 100 101 105 Benzene bands, 39 Benzene bands, 39 Benzene bands, 180 39 Benzene bands, 200 39 Benzene bands, 260 39 Benzene bands, A 39 Benzene bands, C 39 Benzene bands, K 39 Benzene bands, nomenclature 39 Benzene bands, primary (first) 39 Benzene bands, primary (second) 39 Benzene bands, principal 39 Benzene bands, secondary 39 Benzene complexes with iodine 119 120 Benzene rings, bent 89 Benzenes, alkyl-substituted 41 95 Benzenes, monosubstituted 40 Benzenes, p-disubstituted 44 Benzenes, p-halogen substituted 45 Benzenes, substituted 40 47 101 102 Benzenoid absorption 40 Benzensulphonamide 45 Benzil 33 89 Benzils 87 Benzofuraxan 78 Benzoic acid 45 76 143 Benzoic acids 47 Benzoic acids, ortho-substituted 87 Benzoic acids, substituted 144 Benzophenone 19 141 Benzotropylium 134 Benzoylacetone 72 Benzpyrene 100 Benzthiazoles 53 Benzylpenicillin 64 Biacetyl 25 32 101 Bimesityl 87 Biphenyl 103 Biphenyls, disubstituted 84 Biphenyls, ortho-substituted 82 bis-Diphenylenecumulenes 37 Blue-shift 12 118 139 141 Bonding, metal-porphyrin 104 Bonds, 'Essential' double 88 Bonds, multiple 16 Bonds, S-bond 88 Borazole derivatives 50 Bromocyclohexanone 19 Bromoketones, steroid 19 Bromoketones, triterpenoid 19 Butadiene 88 Butylcrotonal-dimine 25 Caffeine 58 Calcium fluoride 104 Calcium hexose diphosphoric acid 104 Calcium tungstate 104 Calomel 104 Camphor 19 Camphorquinone 33 Capri blue 103 Carbanions 134 Carbazole 53 102 Carbohydrates 73 Carbon disulphide 61 Carbonate ions 22 Carbonium ions 134 Carbonyl compounds, 27 65 Carbonyl compounds, derivatives 30 Carbonyl compounds, saturated 17 Carbonyl group 17 Carbonyls 96 Carboxyhaemoglobin 136 Carotene 135 Carotenoid pigments 33 Carotenoid systems 35 Carotenoids 69 135 Carotenoids, all-trans 69 Carotenoids, poly-cis 70 Cedrone 29 Cell holders, thermostated 7 78 143 cells 7 Cells, thermostated 142 Charge transfer 112 114 115 Charge transfer absorption bands 113 141 Charge transfer complexes 113 118—120 Charge transfer complexes, luminescent spectra 111 Charge transfer complexes, spectra 116 Charge transfer complexes, spectral features 113 Charge transfer complexes, structural alignment 120 Charge transfer complexes, theory 114 Charge transfer complexes, thermodynamics 118 Charge transfer forces, orientation 121 Charge transfer forces, range 118 Charge transfer spectra 111 Charge transfer, intramolecular 18 Charge-resonance spectra 134 Chelation 71 104 Chemical reactions, study of 78 Chlorides, double 107 Chlorobenzenes 47 Chlorophyll 73 74 99 135 Chlorophyll a 104 Chlorophyll b 104 Chlorophyll-protein complex 79 Cholesta-3:5-diene 26 Cholestadienol- 27 Cholestadienol-c 26 Chromophores 9 10 16 Chromophores, insulated 36 Chromophores, partial 63 Chrysene 48 cis-2-Alkenes 94 Cis-peak 70 cis-Stilbene 106 Citral 29 Co-ordination complexes, inorganic 139 Colour centres 112 Colour centres in inorganic compounds 131 Colouring principles 134 Complex formation 118 119 Complex resonance theory 114 Complexes, ground state 117 Complexes, heats of formation 113 Complexes, irregular dodecahedral 126 Complexes, molecular 111 Complexes, octahedral 126 Complexes, square planar 126 Complexes, stability 116 Complexes, tetrahedral 126 Conformational analysis 19 Conformations 19 Conjugation 19 20 25 41 Contact charge transfer complexes 117 118 Contact pair 118 Continuum 11 Conversion, internal 100 Coumarions 53 Coupling, between donor and acceptor 117 Crotonaldehyde 25 29 Crystal field theory 124 Crystal violet 88 89 103 Cumulated systems 37 Curve subtraction method 68 Cyanin 135
Реклама
 |
|
О проекте
|
|
О проекте



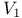 -centres
-centres  -Cyperone
-Cyperone  -2,4-Dinitrophenyl-propionyl esters
-2,4-Dinitrophenyl-propionyl esters  electrons
electrons  electrons
electrons 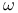 -carboxylic acid
-carboxylic acid 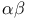 -unsaturated
-unsaturated 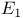
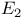
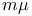
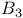 -acetate
-acetate