|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Pauling L., Goudsmit S. — The Structure of Line Spectra |
|
|
 |
| Предметный указатель |
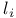 , selection rules for 93 137 , selection rules for 93 137
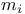 , quantum number 215 , quantum number 215
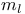 , quantum number 33 75 , quantum number 33 75
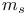 , quantum number 33 75 , quantum number 33 75
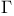 -permanence rule 158 -permanence rule 158
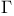 -sum rule 158 -sum rule 158
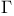 -values for configurations of equivalent electrons 159 -values for configurations of equivalent electrons 159
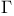 -values for more general configurations 164 -values for more general configurations 164
(jj) coupling 108
(jj) coupling, allowed states with 257
(jj) coupling, fine structure for 125
a priori probability 77
Absorption spectra 1 48
Allowed states with (jj) coupling 257
Allowed states with Russell-Saunders coupling 153
Atomic models 11
Atomic models, potential functions 44
Auroral line, green 96
Azimuthal quantum number k 17
Balmer series of hydrogen 2
Band spectra 1
Barnett effect 238
Bismuth spectrum, hyperfine structure of 211
Bismuth spectrum, Zeeman effect of 217
Bohr correspondence principle 131
Bohr correspondence principle, frequency rule 6
Bohr correspondence principle, magneton 68
Bohr correspondence principle, orbits for hydrogen 19
Bohr correspondence principle, periodic system 147
Boltzmann factor 129
Brackett series of hydrogen 3
Calcium, level scheme for 94
Chemical valence, effect on X-ray edges 194
Chemical valence, relation to ionization potentials 167
Combination principle, Ritz 3
Correspondence principle 131
Coupling of atom core and emitting electron 111
Coupling of atom core and emitting electron, (jj) 108
Coupling of quantum vectors 104
Coupling, Russell — Saunders 107 108
Coupling, types of 104
Curie constant 230
Dirac, theory of electron 54 183
Dirac, transformation theory 10
Doublet, levels 53
Doublet, separations 58
Doublet, separations for X-ray levels 181
Doublet, separations in hydrogen 65
Doublet, separations in isoelectronic sequences 61
Doublet, separations, Lande formula for 60
Dynamical quantities, average values of 31
Eigenfunctions 25
Eigenfunctions for hydrogen-like atoms 27
Einstein transition probabilities 128
Einstein — de Haas effect 238
Einstein's coefficients of absorption and emission 129
Electron collisions, configurations of elements 145 148
Electron collisions, distribution function 30
Electron collisions, distribution function for alkali ions 36
Electron collisions, shells 145
Electron collisions, shells, completed, symmetry of 30
Electron collisions, shells, idealization of 37
Electron collisions, spinning 53
Electron collisions, spinning, Dirac theory of 54 183
Electron collisions, verification of term values by 51
Electrons, equivalent, allowed states for 153 257
Electrons, equivalent, allowed states for, 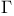 -values for 159 -values for 159
Exclusion principle 144
Exclusion principle, states allowed by 153 257
External screening 180
External screening, quantitative discussion of 187
f, hyperfine quantum number 204
f, selection rules for 205
Fine structure of alkali-like atoms 53
Fine structure of hydrogen and ionized helium 63
Fine-structure constant, Sommerfeld 58
Fine-structure separations, equations for doublets 58 60
Fine-structure separations, equations for many-electron atoms 124 157
Fine-structure separations, equations for triplets 97
Frequency rule, Bohr 6
g-factor of Lande 67 112
g-factor of Lande, tables of 71 115
g-permanence rule 120
g-sum rule 117 124
Heisenberg — Dirac resonance phenomenon 88
Heisenberg, quantum mechanics 8
Heisenberg, uncertainty principle 10
Helium and ionized lithium, spectra of 102
Helium, ionized, fine structure of 63
Hydrogen atom, in quantum mechanics 25
Hydrogen atom, quantum theory of 13
Hydrogen-like atoms, eigenfunctions for 27
Hydrogen-like atoms, fine structure of 63
Hydrogen-like atoms, orbits in 19
Hydrogen-like atoms, quantization of 13
Hydrogen-like atoms, Stark effect of 79 83
Hydrogen-like atoms, stationary states of 13
Hydrogen-like eigenfunctions 27
Hyperfine quantum number f 204
Hyperfine structure, of bismuth spectrum 211
Hyperfine structure, of bismuth spectrum, Zeeman effect of 217
Hyperfine structure, possible explanations of 202
Hyperfine structure, separation formulas for 208
Hyperfine structure, Zeeman effect of 217
i, nuclear spin quantum number 203
Intensity formulas, for multiplets 137
Intensity formulas, for Zeeman effect 142
Intensity of spectral lines 128
Interval rule 124
Inverted terms 161 163
Ion formation and ionization potentials 167
Ionization potentials 51
Ionization potentials and ion formation 167
Ionization potentials, table of 168
Iron, spectrum of 246
Irregular X-ray doublets 178
J, total angular momentum quantum number 55
j, total angular momentum quantum number, selection rules for 56 93 136
k, azimuthal quantum number 17
L, quantum number 27 33
l, quantum number, selection rules for 49 93 134 136
Lande doublet formula 60
Lande doublet formula, g-factor 67 112
Lande doublet formula, g-factor, 67, 112, tables of 71 115
Lande doublet formula, interval rule 125
Laporte rule 94
Lithium, ionized, spectrum of 102
Lorentz unit 70
Lyman series of hydrogen 3
m, magnetic quantum number 33 69
m, magnetic quantum number, selection and polarization rules for 71 135
Magnetic moments, from Stern-Gerlaeh experiment 226
Magnetic moments, of iron-group ions 234
Magnetic moments, of rare earth ions 232
Magnetic nucleus, interaction with extra-nuclear electrons 204
Magnetic quantum number m 33 69
Magneto-mechanical effects 238
Magneton, Bohr 68
Matrix mechanics 8
Metastable states 94
Model, atomic 11 37
Model, interpretation of stationary states in terms of 29
Model, vector for alkali-like atoms 67
Model, vector for many-electron atoms 108
Model, vector for two-electron atoms 87
Model, vector, description of 32
Moseley diagrams 175 184
| Muitiplets 108
Muitiplets, intensity formulas for 137
Multiplet, separations for equivalent electrons 162
Multiplet, separations for general configurations 164
Multiplet, separations, magnitudes of 157
Multiplet, structure in X-ray spectra 192
n', radial quantum number 17
n, principal quantum number 17
Nebular lines, origin of 96
Neon, intensities in 142
Neon, spectrum of 252
Normal states of atoms and ions 148 166
Nuclear moments 221
Nuclear spin quantum number i 203
Nucleus, motion of, in hydrogen-like atoms 20
Optical, spectra, application of X-ray laws to 196
Optical, transition from X-ray spectra to 199
Orbits, Bohr 19
Orbits, non-penetrating 35
Orbits, non-penetrating, term values for 45
Orbits, penetrating 35
Orbits, penetrating, term values for 40
Paramagnetic susceptibilities 229
Paramagnetic susceptibilities of iron-group ions 234
Paramagnetic susceptibilities of rare-earth ions 232
Paramagnetism 229
Paschen series of hydrogen 3
Paschen — Back effect 73
Paschen — Back effect for many-electron atoms 118
Pauli exclusion principle 144
Pauli exclusion principle, states allowed by 153 257
Periodic system of elements 146
Polarizability of atoms and second-order Stark-effect 84
Polarization of atom core 45
Polarization of resonance radiation in a magnetic field 239
Polarization, rules for Zeeman efect 71 133
Potential functions, atomic 44
Principal quantum number n 17
Quantum, mechanics, development of 8
Quantum, theory 7
Quantum, weight 77
Radial quantun mber n' 17
Regular X-ray doublet 178
Relativistic, energy correction 64
Relativistic, X-ray doublets 178
Resonance, energy 87
Resonance, lines 50
Resonance, phenomenon 88
Resonance, potentials 51
Resonance, radiation, polarization in a magnetic field 239
Ritz combination principle 3
Ritz combination principle, term formula 39
Russell — Saunders coupling of quantum vectors 108
Russell — Saunders coupling, allowed states with 153
Rydberg, constant R 21
Rydberg, term formula 39
s, spin quantum number 53
s, spin quantum number, selection rules for 136
Schroedinger wave mechanics 9
Screening constants for main energy term 180
Screening constants for optical doublets 61
Screening constants for optical triplets 101
Screening constants for size of orbits 188
Screening constants for X-ray spin doublets 181
Screening Doublets, X-ray 185
Selection rules for alkali-like atoms 49 133
Selection rules for f 205
Selection rules for j 56 93 136
Selection rules for many-electron atoms 93 136
Selection rules for s 136
Selection rules for Zeeman effect 71 135
Separation formulas for hyperfine structure 208
Series of spectral lines 2 48
Series, Balmer 2
Series, Brackett 3
Series, Lyman 3
Series, Paschen 3
Series, term 39
Series, term, limits of 95 253
Sodium D-lines, Paschen — Back effect of 76
Sodium D-lines, Zeeman effect of 72
Sommerfeld, fine-structure constant 58
Sommerfeld, theory of X-ray fine structure 181
Spatial quantization of alkali-like atoms 70
Spatial quantization of hydrogen atom 20
Spectra, absorption 1 48
Spectra, band 1
Spectra, representative 246
Spectral lines, intensity of 128
Spectral terms, relative positions of 165
Spin doublets, X-ray 181
Spinning electron 53
Spinning electron, Dirac theory of 54 183
Stark effect 78
Stark effect for non-hydrogen-like atoms 85
Stark effect, first-order, hydrogen and ionized helium 79
Stark effect, second-order 83
Stationary states 6
Stationary states, properties and interpretation in terms of model 29
Stern — Gerlaeh experiment 226
Subgroups 174
Summation rules for intensities 137 142
Term diagrams 49
Term diagrams for calcium 95
Term diagrams for iron 246
Term diagrams for neon 253
Term diagrams for sodium 49 57
Term series and term values 39
Term symbols for alkali-like atoms 56
Term symbols for many-electron atoms 91
Term values, verification by electron collisions 51
Terms and energy levels 7
Terms, relative position of 165
Total angular momentum of atom 54
Transformation theory 10
Transition probabilities, Einstein 128
Triplet separations, equations for 97
Two-electron transition 137
Uncertainty principle 10
Valence, chemical, effect of on X-ray edges 194
Valence, chemical, relation to ionization potentials 167
Vector model for alkali-like atoms 67
Vector model for many-electron atoms 108
Vector model for two-electron atoms 87
Vector model, description of 32
Wave equation, solution of, for hydrogen atom 25
Wave mechanics 9
X-ray absorption edges 171
X-ray absorption edges, structure of 193
X-ray laws, application to optical spectra 196
X-ray levels 172
X-ray line spectra 178
X-ray screening doublets 185
X-ray spectra 170
X-ray spectra and optical spectra, relation of 199
X-ray spectra of higher rank 194
X-ray spectra, comparison with alkali-like and hydrogen-like spectra 191
X-ray spectra, multiplet structure in 192
X-ray spectroscopy 171
X-ray spin doublets 181
X-ray term values 176
X-ray term values, interpretation of 180
Zeeman effect for manv-electron atoms 112
Zeeman effect of alkali-like spectra 67
Zeeman effect of hyperfine structure 215
Zeeman effect, intensity formulas for 142
Zeeman effect, normal and anomalous 69
Zeeman effect, transition between weak and strong fields 77 119
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



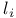 , selection rules for
, selection rules for 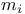 , quantum number
, quantum number 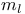 , quantum number
, quantum number 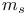 , quantum number
, quantum number 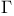 -permanence rule
-permanence rule