Авторизация
Поиск по указателям
Hume-Rothery W. — Electrons, Atoms, Metals and Alloys
Обсудите книгу на научном форуме Нашли опечатку?
Название: Electrons, Atoms, Metals and AlloysАвтор: Hume-Rothery W. Аннотация: The application of electron theory to the structure and properties of metals and alloys has aroused much interest in recent years, but presents great difficulty to the non-mathematical reader. This book is intended for those to whom the ordinary textbook treatment is unattractive. It is divided into four parts dealing with the structure of atoms, metals, alloys and atomic nuclei, and is presented in the form of a dialogue between an Older Metallurgist and a Young Scientist, bringing out clearly the contrast between the old and the new viewpoints. Many difficulties are discussed and the dialogue is framed to give the reader continual help in understanding the problems at issue.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Год издания: 1955Количество страниц: 387Добавлена в каталог: 18.08.2009Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
344 147 152 76 84 93 148 134 134 169 59 ( 182 Absolute Angstrom Unit 16 Acceptable solutions 69 150 action 51 Action, principle of least 51 Aether see "Ether" Alkali metals, 148 Alkali metals, alloys of 284 Alkali metals, compressibilities of 228 Alkali metals, crystal structure of 196 226 Alkali metals, electronic structure of atoms of 32 Alkali metals, melting points of 229 Alkali metals, paramagnetic susceptibility of 249 Alkali metals, theory of 148 150 Alkaline earth metals: melting points of 229 alpha-particles 17 348 365 Aluminium, band spectrum of solid 128 130 Aluminium, deformation of 268 Aluminium, electronic structure of atom of 114 Aluminium, valency of 114 Aluminium-zinc alloys 292 Angstrom unit 15 Angular momentum of electron 29 78 Anti-bonding orbitals 186 Anti-phase domains 342 Antimony-magnesium alloys 308 Antimony-zinc alloys 308 Antimony: crystal structure of 182 190 Argon, cohesion in liquid and solid 178 Argon, electronic structure of atom of 106 108 Arsenic: crystal structure of 182 Artificial isotopes 20 355 Asterism 271 Aston, F.W. 17 19 Atomic diameters 218 Atomic fission 370 Atomic nucleus, charge on 18 Atomic nucleus, dimensions of 18 Atomic nucleus, structure of 348 Atomic number 18 Atomic structure, Bohr theory of 26 Atomic structure, tables of 103 104 105 Atomic unit of length 16 73 Atomic weights: tables, of 111 Avogadro's number 11 15 Avogadro, A. 11 15 Axial ratio 197 Balmer, J.J. 29 Bands, effect of temperature on 129 Bands, electronic energy 127 128 129 130 Barium, melting point of 229 Benzene: structure of 312 Bernal, J.D. 329 Beryllium, electronic structure of atom of 100 Beryllium, ionisation potentials of 100 Beryllium, melting point of 229 Beta-brass: theory of 175 317 322 Beta-manganese Structure: Brillouin zone of 323 Beta-rays 351 Birge, R.T. 15 Bismuth, crystal structure of 182 189 Body-centred cubic structure 195 202 Body-centred cubic structure, Brillouin zone for 167 322 Body-centred cubic structure, deformation of 201 202 260 Bohr magneton 85 94 241 Bohr theory of hydrogen atom 26 Bohr, N. 26 369 Boltzmann's constant 154 226 Boltzmann, L. 154 226 Bonding orbitals 186 Borelius, G. 337 Boron, electronic structure of atom of 101 105 Bradley, A.J. 317 321 331 338 Bragg equation 163 Bragg, W.L. 218 brass see "Copper-zinc alloys" Brillouin zone 166 169 Brillouin, L. 166 169 Cadmium, 233 Cadmium, Brillouin zone for 232 Cadmium, crystal structure of 203 Cadmium, crystals of 121 Cadmium, distortion of 265 Cadmium, melting point of 229 Cadmium-magnesium alloys 291 Cadmium-silver alloys 275 Caesium, 148 Caesium, compressibility of 228 Caesium, crystal structure of 225 227 Caesium, melting point of 229 Caesium-chloride structure 327 Caesium-potassium alloys 285 Caesium-rubidium alloys 285 Calcium fluoride structure 306 Calcium, 230 Calcium, electronic structure of atom of 109 Calcium, melting point of 229 Calcium-strontium alloys 291 Carbon, (Diamond) Crystals of 118 Carbon, crystal structure of 182 Carbon, electronic structure of atom of 102 103 104 Cathode rays 16 Chadwick, J. 357 Channel-Evans, K. 279 290 Chlorine, crystal structure of 180 Close-packed hexagonal structure 197 Close-packed hexagonal structure, Brillouin zone of 231 Close-packed hexagonal structure, deformation of 202 260 265 Co-ordination number 194 Co-valent bonds 179 184 189 Co-valent bonds, in alloys 309 Cobalt, atomic number of 18 Cobalt, atomic weight of 18 Cobalt, crystal structure of 199 Cobalt, magnetic domains in 254 Cockcroft, J.D. 354 Cohen, E.R. 15 Collision radius, effective 350 Compressibility of metals 228 Compton effect 44 Compton, A.H. 44 Conductivity, electrical 217 Copper germanium alloys 286 334 Copper, compressibility of 228 Copper, crystal structure of 225 Copper, deformation of 271 Copper, electronic structure of atom of 109 Copper, melting point of 229 Copper, theory of alloys of 322 Copper-aluminium alloys 290 317 Copper-arsenic alloys 286 Copper-beryllium alloys 280 322 Copper-cadmium alloys 280 Copper-gallium alloys 286 288 299 334 Copper-gold alloys 283 336 Copper-indium alloys 290 301 334 Copper-lead alloys 274 Copper-magnesium alloys 280 343 Copper-mercury alloys 280 Copper-tin alloys 290 300 317 320 Copper-zinc alloys 273 276 280 286 291 294 317 320 322 334 Cosmic rays 360 Cottrell, A.H. 265 creep 259 Crystal Angstrom Unit 16 Crystal faces 117 Crystallites 271 Crystals, defect 122 Crystals, dendritic 119 Crystals, lamellar 121 Crystals, lineage 119 Crystals, mosaic 120 Crystals, perfect 118 119 Crystals, real 119 123 Cubic close packing see "Face-centred cubic structure" Curie, P. 256 d-states 90 Davisson, C. 34 de Broglie, L. 55 de Maupertuis, P.L.M. 51 Decay constant 352 Defect crystals 122 Degeneracy 153 Dendrites 119 Dewar, J. 312 Diamagnetism 246 Diamond, Brillouin zone for 208 Diamond, structure of 183 190 Diffusion 21 122 Dirac, P.A.M. 152 Dislocation hypothesis 261 Domain structure, anti-phase 342 Domain structure, ferromagnetic 253—255 Domain structure, mosaic 120 Du Mond, J.W.M. 15 Ekman, W. 326 Elastic limit 259 Elastic range 259 Electrical resistance 214 257 Electrochemical factor 285 307 333 Electron cloud patterns 80 83 Electron compounds 321—322 Electron concentration 174 287 318 Electron diffraction 35 Electron distribution curves 132 Electron gas 153 Electron in crystals 125 126 Electron waves 34 40 Electron, charge on 16 Electron, discovery of 16 Electron, groups 32 94 Electron, mass of 16 Electron, positive see "Positron" Electron, spin of 93 Electron, thermionic emmission of 16 Electronic charge 16 Electronic energy levels in crystals 126 Electronic energy levels in crystals, effect of temperature on 129 Electronic mass 16 Electronic orbits 24 26 30 Electronic orbits, elliptical 31 Electronic specific heat 154 Electronic spectra 26 Electronic structure of atoms, tables of 103 104 105 Emission spectra 26 Energy contours 169 Ether 37 Exchange forces 188 250 Exchange integral 250 Face-centred cubic structure 193 Face-centred cubic structure, Brillouin zone for 166 295 Face-centred cubic structure, deformation of 201 260 Face-centred-tetragonal structure 206 Faraday, the 16 Fermat's principle 52 Fermat, P. 52 Fermi distribution function 153 Fermi surface 146 Fermi — Dirac statistics 152 Fermi, E. 146 152 Ferricyanide ion 190 ferromagnetism 187 246 250 Fluorine, co-valent bonds in 189 Fluorine, cohesion in solid and liquid 181 Fluorine, crystal structure of 180 Free electron theory 139 149 Free electron theory, of diamagnetism 249 Free electron theory, of paramagnetism 249 Free energy 294 Fuchs, K. 228 Gamma brass 320 324 Gamma phases 320 Gamma phases, Brillouin zone of 325 Gamma rays 351 Geiger, H. 349 Geometrical optics see "Optics" Germanium, crystal structure of 183 Germer, L.H. 34 Gold, compressibility of 228 Gold, crystal structure of 225 Gold, electronic structure of atom of 113 Gold, melting point of 229 Gold-copper alloys 283 336 Gold-magnesium alloys 326 Gold-silver alloys 287 291 Goldschmidt, V.M. 219 223 Goudsmit, S. 93 Gram-atomic susceptibility 247 graphite 214 Gregory, C.H. 321 Group velocity 61 H 26 Haegg, G. 346 Hahn, O. 370 Half-life, time of 352 Hamilton, W.R. 55 Heisenberg's principle 46 63 74 80 143 Heisenberg, W. 46 Helium, electronic structure of atom of 94 Helium, ionisation potential of 95 Helium, liquid 177 Hexagonal close-packing see "Close-packed hexagonal structure" Homopolar bands 179 Hooke's law 259 Hume-Rothery, W. 242 279 290 317 Hybridised orbitals 127 190 Hydrofluoric acid 311 Hydrogen atom, Bohr theory of 26 Hydrogen atom, d states of 90 Hydrogen atom, early theory of 22 Hydrogen atom, electron theory of 22 26 68 75 89 90 Hydrogen atom, ionisation potential of 95 Hydrogen atom, p states of 89 Hydrogen atom, s states of 75 Hydrogen atom, spectrum of 29 Hydrogen atom, structure of 22 Hydrogen atom, wave mechanical theory of 68 75 89 90 Hydrogen molecule, structure of 184 310 Ideal crystals 119 Immiscible liquids see "Liquids" Indium, crystal structure of 206 Indium, electronic structure of atom of 115 Indium, valency of 115 Inert gases, cohesion in liquid and solid 177 Insulators, theory of 210 Intermetallic compounds 303 Interstitial solid solutions see "Solid solutions" Iodine, crystal structure of 179 ionic bond 304 Ionic radii 218 Ionic radii, univalent 219 Ionisation potential 95
Реклама
 |
|
О проекте
|
|
О проекте



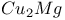 structure
structure 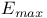
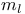
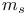
 ) rule
) rule