|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Bijvoet J.M., Kolkmeyer N.H., Macgillavry C.H. — X-Ray Analysis of Crystals |

Обсудите книгу на
Нашли опечатку?
Выделите ее мышкой и нажмите Ctrl+Enter
|
Название: X-Ray Analysis of Crystals
Авторы: Bijvoet J.M., Kolkmeyer N.H., Macgillavry C.H.
Язык: 
Рубрика: Физика/
Статус предметного указателя: Готов указатель с номерами страниц
ed2k:
Издание: 1st edition
Год издания: 1951
Количество страниц: 304
Добавлена в каталог: 09.08.2009
Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
|
|
 |
| Предметный указатель |
Laue diagram diffuse spots in 274
Laue diagram evaluation of 249
Laue indices 23 250
Laue interpretation by 16—21 23
Laue method 5 20 36 249 283
Laue neutron diagram 290
Laue proof 5
Laue symmetry 36 99
Lauric acid 201
Layer line, angle 31—33 244
Layer line, height of 30
Layer line, relation 30—31
Layer structures 97 125 130 134 137 139 142 163 292
Layer structures in random structures 274
Layer structures of the dihalides 163
Lead halides 166
Lead iodide 125 142
Lead iodide type 165
Lead structure 128
Light path 16 21 22 31
Light propagation of 141 199
Light silicates 170
Lindemann glass 27 41
Line width, integral 53
Lines, broadening of 52 57 261
Lithium beryllium fluoride 140
Lithium cyanide 111—112
Lithium ferrite 154
Lithium iodide, isomorphism of hydrated 148
Lithium oxide 168
Lithium perchlorate, hydrated 148
Lithium titanate 154
Long chains 201—209
Lorentz factor 73 74 261
Lubrication 201 203
M-levels 39
Macromolecular compounds 208
Macromolecular compounds amorphous 209
Macromolecular compounds fibrous 209
Macromolecular compounds globular 210
Magma, crystallization of 168 187
Magnesium 128 132
Magnesium carbonate, isomorphism of hydrated 147
Magnesium halides 163
Magnesium lead alloy 168
Magnesium oxide 132 140
Magnesium oxide mixed crystals with  and and  154—155 154—155
Magnesium perchlorate, hydrated 148
Magnesium sulphide, contact of ions in 132
Manganese 128 132 148 189
Manganese carbonate, isomorphism in 147
Manganese sulphide, contact of ions in 132
Margarite 169 179
Mercuric chloride 32 84—94
Mercurous chloride see “calomel”
Mesomerism 197
Metal compounds see “intermetallic C.”
Metal structures 128
Metals 53ff 128
Metals bonds in 128 293
Metals cold working of 56
Metasilicates 169 170 177
Methaemoglobin, horse 220
Methane, rotation in 159
Method, Bragg 21 26 259
Method, Fourier CHap. 5 APp. VII
Method, Hull 243
Method, Laue 20 36 249 260
Method, oscillation 34 259
Method, powder 20 28 241
Method, rotation 30 244 259
Method, Weissenberg 35 81 212 246
Micas, composition of 177—181
Micas, electron diffraction diagram of 281 282
Micas, properties of 137 142 169 177 188
Micas, structure of 172 173 177ff
Micelle structure 209
Miller indices 22 249 252
Minimum wavelength 39 251
Mixed crystal 47—50 149—151 271
Mixed crystal 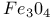 - - 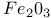 149 149
Mixed crystal 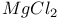 - LiCl 148 - LiCl 148
Mixed crystal types, defect 149
Mixed crystal types, interstitial 149
Mixed crystal types, substitutional 148
Mixture 47 49
Modifications of long chain structures 205
Modulation in spacing 272—273
Modulation of diffracting power 271
Molecular structures 128 136 149 167
Molecule, symmetry of the 98
Molecules, number per cell 80 241
Molybdenum sulphide 162—163
Monochromator 41
Monticellite 184
Montmorillonite 181
Mosaic structure 76 77
Moseley's relation 39
Multiplicity (of equivalent planes) 60 75 242 245
Multiplicity of lattice sites 81 82 155
Multiplicity of rotation 7 8 10
Muscovite 169 178—179 188
naphthalene 98 192 197
Net-plane 8 17 22 25
Net-plane diffraction by 18—19
Net-plane distance 22 24 26 45 236
Net-plane distance standard values 45
Neutron diffraction 289
Nickel 189
Nickel filter 40
Nickel halides 163
Nitrides 137
Nomenclature of classes 85 229—230
Nomenclature of groups 10 14 85 232
Number of equivalent planes see “multiplicity”
Numerical aperture of electron microscope 288
Nylon 209 212
Olivine 96 147 169 171 183 184 186 188
Olivine close oxygen packing in 182 184
Olivine refractive index of 184
Order degree of 149
Order in mixed crystals 152
Order of diffraction (reflection) 17 18 20—23 27 44
Order of diffraction by Laue method 252
Orientation of long chains 201
Orthoclase 174 183 188
Orthosilicates 168 169 177 187
Oscillation photograph 34 97 219 259
Oscillations, composition of 62—67 238
Oxygen, closely packed 132 149 155—182
Oxygen, refractive index of 184
Packing see “close P.”
Paraffins 137 139 143 157—158 201 202 203
Paraffins  205 205
Parameters 16 62
Parameters separation of 82 87
Parameters undetermined see “Undetd. P.”
Particle size see “crystallite”
Passivity 287
Path difference see also “phase D.” 16 17 43 61
Pattern, crystal 4—12
Patterson analysis 96 115 220 APp.
Pauling's rules 95 124 185
Pearls, real and cultured 56
Penicillin 118
Pentaerythritol 200
Perchlorates, rotation isomorphism in 159
Period 182
Period in direction of rotation axis 32
Perovskite 124 140
| Phase diagram, X-ray 50—51
Phase difference 4 61—62 67 238
Phase difference Fourier amplitudes 107ff 265ff
Phase difference of alloys, disordered 47—49 150—156 277
Phenacite 185ff
Philips X-ray apparatus 41
Phosphorus pentoxide 143—144
Phthalocyanins 113 192 198
Physical properties and crystal structure 137—146 155 156 184
Plagioclases 170
Plane, glide 11 12 14 69 232
Plane, groups 8 14
Plane, symmetry (reflection 11 14 69 228—229
Plasticity 128 139 143
Plastics 209
Point lattices 6—15 APp. II
Polar axis 37 81
Polarizability 126 135 141 142 162 165
Polarization factor 72 74 75
Polarization of ions 125
Polish layer 288
Polyamide 216
Polybutadiene 209
Polyethylene 205 210
Polyisobutylene 210
Polyisoprene chains 215 216
Polymorphism 143 147 168 174
Polymorphism of long chain structures 205
Polypeptides 217—219
Polyvinyl derivatives 209 210
Polyvinylidene 210
Porphyrin derivatives 198
Potassium chloroplatinate 82 124
Potassium nickel fluoride 124 140
Potassium niobate 140
Potassium permanganate 147
Potassium sodium crystal compound 150—151
Potential, internal 280
Powder diagram, indexing of 29 83 241
Powder method 20 28
Precious stones, real and artificial 47
Pressing 56 146
Primary extinction 77
Primitive lattice 9 12 16 67
Projection, gnomonic 249
Projection, stereographic 226
Proteins 208 219
Proteins amorphous 209
Proteins fibrous 209 217
Proteins globular 210 219
Pyrite 167
Pyroxenes 171 172
Quadratic function 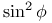 24 24
qualitative analysis 46
Quantitative analysis 46
Quartz 78 147 170 174 175 186
Quinol compounds 193
Radiation,  , L-, M- 38ff , L-, M- 38ff
Radiation, Characteristic 39—40
Radiation, white 39—40
Random structure 274
Reciprocal lattice 248 255
Reciprocal lattice and Fourier expansion 269
Reciprocal lattice demonstration apparatus 284
Reciprocal lattice for electron rays 284 285
Reciprocal lattice of random structures 276—277
Recrystallization 55 57
Reflection conditions, Bragg's 21
Reflection method 26
Refraction of X-rays 45
Refractive index 98 140—142 143 184 199
Resonance (state) 197
Reversed scattering phase 290
Rock salt see “sodium chloride”
Rod diameter, correction for 241
Rolling 54 55 56 146
Rolling texture 54 55 211
Rotation 8—12
Rotation diagram, evaluation of 32 244
Rotation in crystal structures 156
Rotation method 30—34 84
Rotation method layer line relation 32
Rowland grating 43
Rubber 196 208 215
Rubber substitute 209
Rules, Pauling's 95 124 185
Rutile structure 163 166 168
Saccharose 192 193
Saccharose  192 192
Saccharose  192 192
Satellites in reciprocal lattice 272 273
Sauter diagram 35
Scattering see also “diffraction”
Scattering of neutrons 290
Scattering power 69—73 103 242
Scattering power determination of 71—73
Scattering power of electron 72 75
Screw axes 11 12 232
Screw axes absence conditions 68
Screw axes components 68
Secondary extinction 76
Secondary radiation 27
Selenium 130
Separation of parameters see “P.”
Shape of particles 261
Sheet cleavage 96 137 169 170 173 199
Side chain period in polypeptides 218
Signs of Fourier coefficients 108—110 115 264 267
Silicates 5 CHap.
Silicates groups of 169
Silicates isomorphous substitution in 176 179 189
Silicates refraction of light in 96 142 182
Silicates structure determination of 182—186
Silk fibroin 217 219
Silver iodide 156
Silver mercury iodide 155 156 157
Silver mixed crystal with Au 150
Silver selenide 156
Silver structure 128
Silver sulphide 156
Silver texture of rolled sheet 54—55
Single layer structure 165
SOAP 202
Soap influence of water content 205
Sodium 129
Sodium chloride 78 138 144
Sodium chloride deformation 138
Sodium chloride electron density 105—106 118
Sodium chloride indexing of Laue photograph 249
Sodium chloride indexing of powder photograph 242
Sodium chloride indexing of rotation photograph 244
Sodium chloride indexing of Weissenberg photograph 246
Sodium chloride structure type 42 65 123 150
Sodium cyanide 158 159
Sodium hydride and deuteride, neutron diffraction patterns 289
Sodium nitrate 142 147
Sodium nitrate lattice, rotation in 156—157
Sodium potassium alloy 150
Sodium scattering of neutrons by 290
Soil colloids 46
Space groups 11—15 80—95 APp.
Space groups table of 233—235
Space lattice 3 11 19
SPACING see “net-plane distance”
Spacing in polypeptides, long 219
Special absences see “absence”
Spectrometer by Bragg 26
Spectrum, characteristic 39—40
Spectrum, continuous 39
Sphere of reflection 258 283
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 and
and 
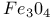 -
- 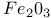
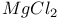 - LiCl
- LiCl 
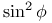
 , L-, M-
, L-, M- 
