|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Cordell G.A. (ed.) Ч The Alkaloids Vol. 55 |
|
|
 |
| ѕредметный указатель |
17-O-Deacetyl-12-methoxyvinorine structure 27
17-O-Deacetyl-21-deoxy-12-methoxyvomilenine 6
19,20-Dihydrovomilenine  data 72 data 72
19,20-Dihydrovomilenine  and mass spectral data 54 and mass spectral data 54
19,20-Dihydrovomilenine plant origin 12
19,20-Dihydrovomilenine structure 32
19-Hydroxy-19,20-dihydrovincamajine  and mass spectral data 56 and mass spectral data 56
19-Hydroxy-19,20-dihydrovincamajine plant origin 14
19-Hydroxy-19,20-dihydrovincamajine structure 33
2-DeshydronortetraphylIicine 9
21-Acetyl-19,20-dihydrovomilenine  and mass spectral data 56 and mass spectral data 56
21-Acetyl-19,20-dihydrovomilenine plant origin 14
21-Acetyl-19,20-dihydrovomilenine structure 34
21-Deoxyvomilenine 9
3-Butyl-5-propylindolizidines 165
4'-Hydroxy-3',5'-dimethoxybenzoylvincamajine  and mass spectral data 60 and mass spectral data 60
4'-Hydroxy-3',5'-dimethoxybenzoylvincamajine plant origin 16
4'-Hydroxy-3',5'-dimethoxybenzoylvincamajine structure 37
4,6-Dialkylquinolizidine alkaloid 195C 175
5-Alkylindolizidine alkaloids 177Ч186
6,7-Diepicastanospermine 138
A58365A 105Ч108
A58365B 105Ч108
Ajmalidine group, mass spectrometry 67Ч68
Ajmaline alkaloids  65Ч66 70Ч78 65Ч66 70Ч78
Ajmaline alkaloids  47Ч65 47Ч65
Ajmaline alkaloids biogenesis 44Ч46
Ajmaline alkaloids biosynthesis 44Ч46
Ajmaline alkaloids derivatives 2
Ajmaline alkaloids early reviews 1Ч2
Ajmaline alkaloids mass spectrometry 47Ч64 66Ч69
Ajmaline alkaloids pharmacology 79
Ajmaline alkaloids plant origins 3Ч18
Ajmaline alkaloids ring system 2
Ajmaline alkaloids structures 2
Ajmaline group 66Ч67
Ajmaline in (-)-20-hydroxydihydrorankinidine synthesis 43
Ajmaline, Masamune synthesis 19
Ajmaline, Mashimo synthesis 19Ч21
Ajmaline, Sato synthesis 19Ч21
Ajmaline, transformation into raumacline 43
Ajmaline, van Tamelen synthesis 22Ч24
Allopumiliotoxins 203Ч210
Alstonia 9Ч18
Amphibians 1,4-disubstituted quinolizidine alkaloids 194Ч203
Amphibians 3,5-disubstituted indolizidine alkaloids 187Ч193
Amphibians 5,8-disubstituted indolizidine alkaloids 194Ч203
Amphibians 5-alkylindolizidine alkaloids 177Ч186
Amphibians allopumiliotoxins 203Ч210
Amphibians homopumiliotoxins 203Ч210
Amphibians indolizidine alkaloids 175Ч177
Amphibians pumiliotoxins 203Ч210
Amphibians quinolizidine alkaloids 175Ч177
Anti-inflammatory effects 142
Antitumor agents 142
Ants indolizidine alkaloids 165Ч174
Ants quinolizidine alkaloids 165Ч172
Apocynaceae 3Ч18
Aspidosperma 6 12
Astragalus 117
Astragalus polycanthus 146
Beaver indolizidine 236
Biogenesis, ajmaline alkaloids 44Ч46
Biomimetic semisynthesis, alstomacroline and alstonisidine 24Ч25
Biosynthesis ajmaline alkaloids 44Ч46
Biosynthesis cyclizidine 100Ч102
Biosynthesis swainsonine 118Ч119
Biotechnology, swainsonine 118Ч119
Bongardia chrysogonum, (+)-17-desoxycis-lamprolobine 153
Cabucala, ajmaline alkaloids 12Ч13
Cancer, swainsonine chemotherapy 130
Castanospermine biological activity 140Ч142
Castanospermine chemical transformation 139
Castanospermine isolation 130Ч131
Castanospermine structure 131
Castanospermine synthesis 131Ч138
Castanospermine-related alkaloids 130Ч142
Castanospermum australe, castanospermine 130Ч131
Cell cultures, swainsonine effects 129
Chemical transformation ajmaline into raumacline 43
Chemical transformation castanospermine 139
Chemical transformation vomilenines into perakines 42Ч43
Chemotherapy, with swainsonine 130
Clathyrimines 231
Clavelina picta, alkaloid 217
Clavepictine A 218Ч219
Clavepictine B 218Ч219
Comins dihydropyridone method 163
Cook synthesis, (+)-ajmaline 21Ч22
Cyclizidine 100Ч102
D-Glucopyranosides 139
Dehydroepilupinine 149
Dihydrolusitanine 152
Dihydronorpurpeline  and mass spectral data 49 and mass spectral data 49
Dihydronorpurpeline plant origin 6
Dihydronorpurpeline structure 28
Elaeocarpus, indolizidine alkaloids 143
Endolobine  and mass spectral data 48 and mass spectral data 48
Endolobine plant origin 5
Endolobine structure 27
Epilupinine esters 150
Epimyrtine 159Ч161
Esters, swainsonine-based 130
Ficuseptine 229
Fritillaria maximowiczii 147
Fungal alkaloids 94Ч99
Glucosidase 140Ч141
Glycosidase 129
Halichlorine 212Ч213
Halichondria okadai, halichlorine 212
Herpes simplex virus type 1, castanospermine effects 141
Herpes simplex virus type 2, castanospermine effects 141
Homopumiliotoxins 203Ч210
Human immunodeficiency virus type 1, castanospermine effects 141
Hydroxylated indolizidine alkaloids 1,2-dihydroxyindolizidines 112Ч117
Hydroxylated indolizidine alkaloids 1-hydroxyindolizidines 109Ч112
Hydroxylated indolizidine alkaloids swainsonine and related compounds 117Ч130
Immunomodulatory effects, swainsonine 128Ч129
Immunosuppression, by castanospermine 142
Indolizidin-3-one 178 181Ч182
Indolizidine 167B 178Ч183
Indolizidine 195B 187Ч188
Indolizidine 209B 196Ч198
Indolizidine 209D 183Ч185
Indolizidine 223AB 187 190Ч193
Indolizidine alkaloids from amphibians, 3,5-disubstituted alkaloids 187Ч193
Indolizidine alkaloids from amphibians, 5,8-disubstituted alkaloids 194Ч203
Indolizidine alkaloids from amphibians, 5-alkylindolizidine alkaloids 177Ч186
Indolizidine alkaloids from amphibians, isolation, structure, and biological activity 175Ч177
Indolizidine alkaloids from ants 165Ч172 173Ч174
Indolizidine alkaloids from Astragalus polycanthus 146
Indolizidine alkaloids from Elaeocarpus 143
Indolizidine alkaloids from Polygonatum sibiricum 146
Indolizidine alkaloids from Prosopis 144Ч146
Indolizidine alkaloids from tunicates 217Ч219
Indolizidinone 143
Indolizomycin 102Ч104
Ipalbidine isolation 221Ч222
Ipalbidine synthesis 223Ч224
Ipalbidinium 222
Ipalbine 221Ч222
| Ipomoea alba, ipalbidine and ipalbine 221Ч222
Isoajmaline Mashimo synthesis 19
Isoajmaline Sato synthesis 19
Isoipomine 222
Isosaraines 214Ч216
Julandine 229Ч230
Julifloricine 144Ч145
Juliflorinine 145Ч146
Juliprosine 144
Juliprosinene 145Ч146
Juliprosopine 144
L-Proline, to (-)-8a-epidendroprimine 147
Lasubines 231Ч237
Lentiginosine 112Ч117
Lipoprotein lipase 141
Louludinium chloride 211
Lupine alkaloids epilupinine 153Ч155
Lupine alkaloids epimyrtine 159Ч161
Lupine alkaloids isolation and structure 148Ч153
Lupine alkaloids lupinine 153Ч155
Lupine alkaloids myrtine 159Ч161
Lupine alkaloids tashiromine 156Ч158
Lupinine 153Ч155
Lupinus hirsutus 148
Lyngbya gracilis, louludinium chloride 211
Lythraceous alkaloids, biosynthetic studies 231Ч237
Maackia amurensis, (+)-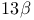 -hydroxymamanine 153 -hydroxymamanine 153
Maackia hupehensis, (+)-hupeol 148
Majdinine, plant origin 14
Majorinine  and mass spectral data 55 and mass spectral data 55
Majorinine plant origin 14
Majorinine structure 33
Masamune synthesis, ajmaline 19
Mashimo synthesis ajmaline 19Ч21
Mashimo synthesis isoajmaline 19
Mass spectrometry, ajmaline alkaloids 47Ч64 66Ч69
Melodinus, ajmaline alkaloids 6
Metarhizium anisopliae, swainsonine 117
Microbial alkaloids A58365A and A58365B 105Ч108
Microbial alkaloids cyclizidine 100Ч102
Microbial alkaloids indolizomycin 102Ч104
Moloney murine leukemia virus 141
Mucor javanicus, castanospermine effects 140
Myrmicaria eumenoides, indolizidine alkaloids 166
Myrtine 159Ч161
Newcastle disease virus, castanospermine effects 141
Normitoridine  and mass spectral data 48 and mass spectral data 48
Normitoridine plant origin 5
Normitoridine structure 26
Norrauvomitine  and mass spectral data 59 and mass spectral data 59
Norrauvomitine plant origin 16
Norrauvomitine structure 36
Nortetraphyllicine  and mass spectral data 47 and mass spectral data 47
Nortetraphyllicine plant origin 4
Nortetraphyllicine structure 26
Norvincamajine N(1)-tri-O-methylgallate 17
Nuclear magnetic resonance  , ajmaline alkaloides 65Ч66 70Ч78 , ajmaline alkaloides 65Ч66 70Ч78
Nuclear magnetic resonance  , ajmaline alkaloids 47Ч65 , ajmaline alkaloids 47Ч65
Nuphar indolizidine 236
Oxidation, vincamajine to voachalotinal 44
Oxytropis, swainsonine 117
Perakines, from vomilenine transformation 42Ч43
Petrosins 214Ч216
Pharmacokinetics, swainsonine 129Ч130
Pharmacology, ajmaline alkaloids 79
Plumerinine 162
Polycanthisine 146
Polygonatum sibiricum, indolizidine alkaloids 146
Poranthera corymbosa, alkaloids 162Ч164
Porantherilidine 162Ч163
Prosopis juliflora, indolizidine alkaloids 144Ч146
Pumiliotoxin 251D 204
Pumiliotoxin 307A 205
Pumiliotoxins 203Ч210
Quebrachidine group, mass spectrometry 69
Quinolizidine alkaloids from amphibians, 1,4-disubstituted alkaloids 194Ч203
Quinolizidine alkaloids from amphibians, isolation, structure, and biological activity 175Ч177
Quinolizidine alkaloids from ants 165Ч172
Raucaffriline  and mass spectral data 53 and mass spectral data 53
Raucaffriline plant origin 11
Raucaffriline structure 31
Raucaffrine 10
Raugalline 6
Raumacline, from ajmaline transformation 43
Rauscher murine leukemia virus, castanospermine effects 141
Rauvolfia, ajmaline alkaloids 4Ч17
Rauwolfine 6
Rhazya, ajmaline alkaloids 10
Rhizoctonia leguminicola, slaframine 94Ч99
Rhizoctonia leguminicola, swainsonine 117
Saraines 214Ч216
Sato synthesis, ajmaline 19Ч21
Sato synthesis, isoajmaline 19
Secophenanthroindolizidine alkaloids 225Ч228
Semperfiorine 4
Septicine 225Ч228
Serpinine 4
Simian immunodeficiency virus, castanospermine effects 141
Slaframine 94Ч99
Stellettamides 211Ч212
Streptomyces A58365A and A58356B 105Ч108
Streptomyces cyclizidine 100Ч102
Streptomyces indolizomycin 102Ч104
Swainsona, swainsonine 117
Swainsonine biological activity 127Ч130
Swainsonine biosynthesis 118Ч119
Swainsonine biotechnology 118Ч119
Swainsonine distribution 118
Swainsonine isolation 117
Swainsonine structural studies 118
Swainsonine synthesis 119Ч127
Swainsonine-related alkaloids 117Ч130
Tabernaemontana, ajmaline alkaloids 12
Tashiromine source 152
Tashiromine synthesis 156Ч158
Therapeutic agents D-glucopyranosides 139
Therapeutic agents with swainsonine 129Ч130
Tonduzia longifolia, ajmaline alkaloids 8
Tonduzia, ajmaline alkaloids 13 18
Toxicology, swainsonine 128
Transformation ajmaline into raumacline 43
Transformation castanospermine 139
Transformation vomilenines into perakines 42Ч43
Tunicates, alkaloids 217Ч219
van Tamelen synthesis, ajmaline 22Ч24
Vinca, ajmaline alkaloids 9 10 12Ч15
Vincamajine 17-O-veratrate 16
Vincamajine acetate 15
Vincamajine, oxidation to voachalotinal 44
Vincamajinine  and mass spectral data 55 and mass spectral data 55
Vincamajinine plant origin 14
Vincamajinine structure 33
Vincamajoreine  data 71 data 71
Vincamajoreine  and mass spectral data 51 and mass spectral data 51
Vincamajoreine plant origin 9
Vincamajoreine structure 30
Voacanga, ajmaline alkaloids 10
Voachalotinal, from vincamajine oxidation 44
Vomilenines, transformation into perakines 42Ч43
Willicourtine 16
Z-Vomilenine 11
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 data
data  and mass spectral data
and mass spectral data 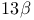 -hydroxymamanine
-hydroxymamanine  , ajmaline alkaloides
, ajmaline alkaloides  , ajmaline alkaloids
, ajmaline alkaloids