|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Jacobsen E.N.N. (ed.), Pfaltz A. (ed.), Yamamoto H. (ed.) — Comprehensive Asymmetric Catalysis. Supplement 2 |
|
|
 |
| Предметный указатель |
 -Methoxyacetophenone 14 -Methoxyacetophenone 14
 -Methyl styrene, asymmetric dihydroxylation 31 -Methyl styrene, asymmetric dihydroxylation 31
 -Ketoesters 129 -Ketoesters 129
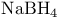 14 14
1,3-Diketones, stereoselective reduction 14
1,3-Diphenylprop-2-enyl acetate 77
1,3-Diphenylprop-2-enyl ethylcarbonate 80
1,3-Diphenylpropenyl ester 75 76
1-Ethyl-3-methylimidazolium tetrafluoroborate 34
1-Indanones 129
2,5-Cyclohexadienylsilanes, AA 68
2-Alkyl-1,3-diketones 16
2-Amino-1,2-diphenylethanol 66
2-Methyl-3-phenyl-2-butene, dihydroxylation 26
2-Naphthoate 100
2-Vinylfuran 61
3-Ethylcyclohexanone 110
4-Hydroxy-2-butenoates 50
Acetyl pyridines 9
Acyloxyborane, chiral 100
AD/N-oxidation 33
Adrenergic receptor agonists 59
Aflatoxin B lactone 84 85
Alcohol-nickel catalysts, bidentate 114
Alcohols, homoallylic 50
Alcohols, nonracemic tertiary homoallylic 105
Alcohols, secondary 7 8
Aldehydes, allyltributyltin, enantioselective addition 101
Aldehydes, enantioselective allylation 100
Alkene, silica-anchored tetrasubstituted 31
Alkenes,  -aminoalcohols 44 -aminoalcohols 44
Alkenes, AD, ligands 23
Alkenes, Baylis-Hillman 25
Alkenes, DHQD ligands 24
Alkenes, enantioselective hydrogenation 4
Alkenes, substituted, AA 49
Alkenes, unfunctionalized 1
Alkenes, zirconocenes 2
Alkylation 109
Alkylation, allylic 73
Allenyltributylstannane, aldehydes 104
Allylamine, lithiated 121
Allylation 73 97
Allylation, Lewis base catalysts 103
Allylic alcohols, Y-fluoroalkylated 83
Allylic substitution 73
Allylsilanes 97
Allylstannates 97 104
Allyltributyltin, enantioselective addition to aldehydes 101
Allyltrichlorosilanes 103 104
Amastatin 64
Amide enolates 127
Amino acids, derivatives 83
Amino acids, protected 79
Amino alcohols 13
Amino, chiral 56
Aminocyclohexitols 66
Aminohydroxylation, asymmetric (AA) 43
Aminohydroxylation, asymmetric (AA), diastereoselectivity 67
Aminohydroxylation, asymmetric (AA), procedures 45
Ammonium enolates, catalytic protonation 130
Anthraquinone 22
Aryl esters, reversal of regioselectivity 47
Arylacrylates, heteroaromatic 56 58
Arylation, rhodium-BINAP-catalyzed 118
Arylglycines 61
Aryllithium 120
Arylmetallic reagents, rhodium-catalyzed arylation 117
Atropisomers, non-biaryl 77
Azetidinone 63
Barbituric acid 82
Baylis-Hillman alkenes 25
Baylis-Hillman, aminohydroxylation, diastereo-selective 68
Benzylamine 76
Benzylic alcohol 48
Benzylic amide 48
Bestatin 64
Bis(N-tosylamino)phosphines 85
Bis(oxazolinyl)phenyl rhodium(III) 101
Bisalkaloid ligands 38
Bisphosphine, copper-catalyzed organozinc addition 113
Borane reagents 7 8
Borohydride 7
Brintzinger-type zirconocene cpmlexes 1
Bronsted acids, chiral 105
BSA 79 80 88
Callipeltoside A 86
Carbamate-AA 51
Carbanucleosides 89
Carbene, chiral heterocyclic 1
Carbonyl compounds, allylic tin reagents 105
Carbonyl compounds, asymmetric allylation 97 100
Carbonyl compounds, Lewis base catalyzed 103
Catecholborane 12
CBS reduction 7 8
Chalcone, diethylzinc 115
Chemoselectivity, AA process 54
Chloramine-T 46
Cinchona alkaloids 21-26 43 47 51
Cinchona alkaloids, AD, silica gel-supported 37
Cinnamates 47 55 56
cis-Alkenes 22
cis-Vinyl epoxides 103
Cobalt complexes,  -keto iminato 14 -keto iminato 14
Conjugate addition 110
Cooxidants 27 44
copper 109
Copper triflate 110
Crabtree-type complexes 1
Crotyltrichlorosilanes 103
Cyclic substrates, Pd-catalyzed 81
Cycloalkenyl series 80
Cyclohexadienone 113
Cyclohexenone 111 112
Cyclomarin A 60
Cyclopentadienylruthenium 80
Cyclopentobarbital 82
Cyclophellitol 82
Dehydrotubifoline 82
Diethylzinc 111
Dihydroquinine (DHQ) 30
| Dihydroxylation, asymmetric (AD) 21 44
Dihydroxylation, asymmetric (AD), molecular oxygen 26
Dihydroxylation, asymmetric (AD), pH 27
Dihydroxylation, catalytic cycles, homogeneous conditions 24
Dimethyl malonate/BSA 79 80 88
Dimethylzinc 110
Diphenylphthalazine 22
Diphenylpyrazinopyridazine 22
EndotherinA 121
Enoates, arylation 120
Enoates, prochiral 125
Enoates, protonation 125 127
Enones, acyclic, Ni/Cu-catalyzed 114
Enones, cyclic 110
Esters,  , ,  -unsaturated 50 -unsaturated 50
Esters, conjugated 62
Ethambutol 86
Exochelin 60
Fenchone 77
Flavin hydrogenperoxide 27
Galanthamine 82
Heck/AD 33
Hydroboration 7
Hydroboration, metal complexes, catalysis 12
Hydrogenation, zirconocene-catalyzed 2
Imidazolylidine, oxazoline 3
Imides, enolates 128
Iridium 73 87
Iridium complexes, chiral 1 3
Isopropyl cinnamate 55
Ketene silyl acetal 78
Ketenes, pyrroles 130
Ketones,  -hetero substituted aliphatic 8 -hetero substituted aliphatic 8
Ketones, aromatic 12
Lactacystin 65
Lewis acid/base catalysts, allylation 100 103
Ligands, chiral, immobilization 34
Ligands, N, S- 78
Ligands, P, N- 75
Ligands, PHAL 50 62
Lithium enolates 127
Loracarbef 64 66
Malyngolide 86
Medermycin 65
MeO-PEG 38
Metal enolates, protonation 125
Methylstilbene 5
Molybdenum 73 86
Mop 79
N-Bromoacetamide 46
N-Chloro-N-metallosulfonamides 44
N-Methylmorpholine N-oxide (NMO) 27
Naphthylacrylate 57
Nickel boride 10
Nitroalkenes 109
Nitroalkenes, copper-catalyzed 116
Nitrogen source, AA reaction 45
Olefins, activated, conjugate addition 119
Olefins, electrophilically activated 109
Organolithium 109
Organolithium reagents, external chiral ligands 120
Organometallic reagents, chiral 122
Organozinc 109
Organozinc reagents, copper-catalyzed conjugate addition 110
Osmaazetidine 53
Osmium azaglycolate 53
Osmium tetroxide 21 43
Osmium(VI) bis(glycolate) 25
Osmium, immobilization 30
Oxazaborolidines, hydroboration catalyzed 7
Oxazaborolidines, immobilization 10
Oxazolines, chiral, arylation 120
Palladium 73
Palladium-catalyzed substitution, mechanism 74
PHAL ligands 50 62
Phenylisoserine 25
Phenyllithium 121
Phosphine 109
Phosphine/oxazoline complexes, iridium 3
Phosphinite, oxazoline 3
Phosphinopyrrolyl, oxazoline 3
Phosphonates, unsaturated 57
Phosphoramidite 110
Phosphoramidite, Taddol-based 115 116
Phosphorus 109
Phthalazine 22
Pinacol 102
Polyketide synthesis 100
Potassium ferricyanide cooxidant 27
Propargyltrichlorosilane 103
Propiophenone 13
Protonation 125
Pyrimidine 22
Pyrrolidines 88
Quinuclidine 35
Radical addition, external chiral ligands 119
Rhodium 118
Rhodium BINAP-catalyzed arylation 118
Rhodium, cationic chiral 102
Ruthenium 73
Samarium enolate 128
Selenoxides 29
Serine, dihydroxyphenyl, fluorinated 59
Silver(I) catalyst, chiral 102
Styrenes 5 47 61
Tetraallyltin 105
Tetralone enolate 128
Tetralones 90 128 129
Tetraponerines 89
Titanium complexes, chiral 100
Titanocene complex 2
trans-Stilbene, AD 35 36
Trioxoimidoosmium(VIII) 52
Tubifoline 82
Vanadyl acetylacetonate 28
Vigabatrin 86
Vinylglycidols 86
Vitamin E 86
Zankiren 64
Zinc enolate 79
Zirconocene complexes, Brintzinger-type 1
Zirconocene complexes, chiral cationic 2
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -Methoxyacetophenone
-Methoxyacetophenone  -Ketoesters
-Ketoesters