|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Taticchi A., Frinquelli F. Ч The Diels-Alder Reaction: Selected Practical Methods |
|
|
 |
| ѕредметный указатель |
Diels Ч Alder reactions of acylnitroso compounds 172t
Diels Ч Alder reactions of surfactant reagents 174Ч176
Diels Ч Alder reactions, base-catalyzed 190
Diels Ч Alder reactions, biocatalyst-promoted 180Ч185
Diels Ч Alder reactions, catalyzed 144 185Ч190 261Ч267
Diels Ч Alder reactions, cationic 128Ч130
Diels Ч Alder reactions, consecutive 2 20 21
Diels Ч Alder reactions, diastereoselective 199 244 255t
Diels Ч Alder reactions, domino 2 20 198
Diels Ч Alder reactions, enantioselective 135 289
Diels Ч Alder reactions, high pressure 205Ч249 267
Diels Ч Alder reactions, inorganic solid-surface-promoted 143Ч149
Diels Ч Alder reactions, ionic 5Ч10 192 200 295
Diels Ч Alder reactions, Lewis acid catalyzed 99Ч142
Diels Ч Alder reactions, micelle-promoted 174Ч179
Diels Ч Alder reactions, microwave-assisted 158Ч163 195 196
Diels Ч Alder reactions, multiple 20Ч22
Diels Ч Alder reactions, normal 122Ч123
Diels Ч Alder reactions, pericyclic 4Ч5 12
Diels Ч Alder reactions, photo-induced 163Ч169
Diels Ч Alder reactions, radical 5Ч10
Diels Ч Alder reactions, regioselectivity of 12 22 23Ч24 148 175 176 198 288t
Diels Ч Alder reactions, repetitive 245
Diels Ч Alder reactions, retro 15Ч18 35 261 290
Diels Ч Alder reactions, stereoselectivity 24Ч25
Diels Ч Alder reactions, tandem 20 21
Diels Ч Alder reactions, thermal 5 29Ч98 162 176 214
Diels Ч Alder reactions, ultrasound-assisted 154Ч158 195
Diels Ч Alder reactions, uncatalyzed 252Ч261
Diels Ч Alder reactions, using resin-anchored reagents 149Ч153
Diels-Alderase 181 184
Dienes 2 3 15 16 25 148
Dienes, across-ring 64Ч66
Dienes, acyclic 36 102
Dienes, heterocyclic 40Ч42 110Ч112
Dienes, inner-outer ring 49Ч64 219Ч223
Dienes, inner-ring 191 223Ч236
Dienes, open chain 29Ч37 191 208Ч217
Dienes, outer-ring 43Ч48 217Ч219
Dienes, stereochemistry of substituents 24
Dienes, sulfinyl groups and 112Ч114
Dienes, surfactant 176
Dienes, tropones as 226Ч229
Dienes, unsymmetric 10 23
Dienophiles 3Ч4 5 8 12 15 44 67 101
Dienophiles, acetylenic 43 49 57
Dienophiles, carbonyl-containing 109 115
Dienophiles, heterocyclic 106Ч108
Dienophiles, imino 137
Dienophiles, olefinic 43
Dienophiles, reactions with dienes 191
Dienophiles, reactivity of neutral 9
Dienophiles, stereochemistry of 25
Dienophiles, sulfinyl groups and 112Ч114
Dienophiles, surfactant 176
Dienophiles, unsymmetric 10 23
Diethyl fumarate 170 173 285
Diethyl maleate 285
Diethylaluminum chloride 126
Dihydro-4-pyridones 187
Dihydrocannivonine 262
Dihydropyranes 123 124
Dihydropyranones 37
Dihydropyrans 122
Dihydropyridines 239
Dihydropyridinones 240
Dihydropyrones 90
Dihydrothiopyrans 123
Dihydrovinylphenanthrenes 55 221
Diiodosamarium 110
Diisopropylethylamine 224
Dimethyl formamide 191
Dimethyl fumarate 8 32 43 44 99
Dimethylacetylenedicarboxylate 32 34 40 49 50 111 127 159
Dimethylaluminum selenide 71
Dimethylamine 69 79
Dimethylaminobutadiene 31
Dimethylcyclobutane 102
Dimethylcyclopropane 102
Dimethylmaleate 32 44 279
Dimethyltetrahydroindenone 223
Dipyridyltetrazine 81
Diterpenes 154 212 232
Divinylbenzene 115
Divinylnaphthalenes 52
Dodecane sulfonates 177
Dodecyl maltoside 174
Dodecyl sulfates 177
Electrogenerated acid (EGA) 192
Electron-withdrawing groups 34 71
Enaminoketones 69 240
Enantioselection 117 135 243 289
Endo adducts 171 174 177 184 190 191 208 209
Endo diastereoselectivity 36 117
Endo selectivity 74 83 192 228 266 276
Endo/exo diastereoselectivity 15 178 179 236 280 288
Enolethers 124 126 237
Epibatidine 90 238
ethane 284
Ethene 4 5 12 29 62
Ethyl acetylenedicarboxylate 260
Ethyl acrylate 32 170 279 285
Ethyl-4-methyl-3,5-hexadienoate 255
ethylene 29 284
Ethylene glycol 278
Ethylpropiolate 34
Ethylvinylether 208
Ethyne 172 227
Ethynyltributyltin 68 91
Exo addition 14 63 145
Exo adducts 15 40 174 184 228 276
Facial stereoselectivity 49 73 83 292
Feringa-butenolide 74
Ferrocenium hexafluorophosphate 114
Flavones 85 89
Fluoboric acid 187
Fluorophenols 33
FMO theory 12 15 22 23 24 57
Formaldehyde 261
Friedel Ч Crafts reactions 279
Fullerene [C60] 36 84 87 168 224 241
Fullerene [C60], condensed aromatics and 193 200
Fullerene [C60], derivatization of 35 67
Fullerene [C60], o-quinodimethanes and 46 47
Fumaric acid 149
Fumaronitrile 8
Functional groups, protection of 252
Furan 57 113 148 170 252 269
Furanamide 272
Furanones 40
Furans 40 58 89 112 229Ч234 267
Furfuryl fumarates 239
Furylaldehydes 149 151
Glucopyranosil-1,3-pentadiene 260
Glyoxal 158 258
Glyoxylic acid 185 258 264 265 294
graphite 160 161 196
GrubТs ruthenium initiator 152
Guanidinium chloride 252 253
Helicenebisquinones 219
Helicenes 55 56
Hematoporphyrin 163 169
Hetero-Diels Ч Alder reactions 34 66Ч73 83 122Ч126 158
Hetero-Diels Ч Alder reactions in supercritical 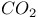 287 287
Hetero-Diels Ч Alder reactions with heterodienophiles 213Ч214
Hetero-Diels Ч Alder reactions, asymmetric 131 133 238
Hetero-Diels Ч Alder reactions, intermolecular 240
Hetero-Diels Ч Alder reactions, intramolecular 79 82 171 292
Heterocycles 57 72 82 149 213
Heterodienes 66Ч70
| Heterodienophiles 66 70Ч73 213Ч217
Hexadecyltrimethylammonium bromide 282
High-speed vibration milling (HSVM) 193
Homo 22 29 57 62 67 107
Homo-Diels Ч Alder reactions 18Ч20 126Ч128
Homobarrelenones 226 227
Hydrindanones 101
Hydrobenzosuberone 76 101
Hydrofluorenones 104
Hydrophenanthrenones 212
Hydroxamic acid 257
Iceane 81
Imino Diels Ч Alder reactions 134 270
Indanone 53 227
Inden-1-one 53 56 221
Indeno[c]phenanthrenones 53
Indium trichloride 134 266 293
Indole 44 63 164
Indolquinolines 9
Intermolecular Diels Ч Alder reactions 1 3 116 205 240 278
Intermolecular Diels Ч Alder reactions, aqueous 290
Intermolecular Diels Ч Alder reactions, Lewis acid catalysis 128
Intramolecular Diels Ч Alder reactions 1 3 8 74Ч83
Intramolecular Diels Ч Alder reactions of amino acid-derivative trienes 149
Intramolecular Diels Ч Alder reactions of franj-cycloalkenones 91
Intramolecular Diels Ч Alder reactions of furans 170 197 232
Intramolecular Diels Ч Alder reactions of furfuryl fumarates 239
Intramolecular Diels Ч Alder reactions of polyenones 270
Intramolecular Diels Ч Alder reactions, high pressure 233
Intramolecular Diels Ч Alder reactions, immonium ion based 291
Intramolecular Diels Ч Alder reactions, microwave-assisted 163
Intramolecular Diels Ч Alder reactions, tandem photooxidation 196
Inverse electron-demand Diels Ч Alder reactions 3 4 23 123Ч125 126 208
Inverse electron-demand Diels Ч Alder reactions, 2-pyrones and 234
Inverse electron-demand Diels Ч Alder reactions, heterocycles and 68
Inverse electron-demand Diels Ч Alder reactions, intermolecular 216
Inverse electron-demand Diels Ч Alder reactions, Lewis acid catalyzed 109
Iodine 191
Ionic liquids 278Ч281
Iron (III) 2-ethylhexaonate 124
Isomerization 14 107 279
Isooctane 282
Isoprene 6 104 108 115 187 287 288
Isoprene, 4-isopropyl-2-cyclehexenone 102
Isoprene, but-3-en-2-one and 279
Isoprene, catalysts and 118
Isoprene, dimethyl acetylenedicarboxylate and 274
Isoprene, maleic anhydride and 286
Isoprene, Nafion-Hand 189
Isoprene, quinidine-5,8-dione and 106
Isoprene, zeolites and 148 194
Isoquinoline-5, 8-dione 106
Isoquinolines 70 106 197
Jatropholone A and B 232
Jencks postulate 184
JV-phenylmaleimide 80 195 226 236 245 276
JV-phenylmaleimide, 1-oxa[4.4.4]propella-5, 7-diene and 224
JV-phenylmaleimide, Lewis acid catalysis 73
JV-phenylmaleimide, methyl palustrate and 243
JV-phenylmaleimide, microwave-assisted reactions 161
K-10 montmorillonite 143 144 145 146 161
Ketals 271 274
Ketodeoxyheptulosonic acid 259
Ketones 63 109 271 274
L-abrine 266
l-menthylallyl-ether 38
Lactams 191 200
Lactones 45 271
Lanthanide shift reagent-catalysis 126
Lanthanide triflates 108 109 110 251 262 264 293
LawessonТs reagent 69
Lewis acid catalysts 37 206 209 214 268 288
Lewis acid catalysts, alkoxybutadienes and 73
Lewis acid catalysts, exo-endo diastereoselectivity 15
Lewis acid catalysts, high pressure and 205
Lewis acid catalysts, norbornene derivatives and 38
Lewis acid catalysts, olefinic acetals and 199
Lewis acid catalysts, quinone-mono-ketals 212
Lewis acid catalysts, surfactant combined 176 177
Lewis acid catalysts, zeolites and 148 194
Lewis acids 23 24 99Ч142 191 230
Lewis acids in ionic liquids 279Ч281
Lewis acids, (-)-menthol-aluminum 147
Lewis acids, coupling photolysis 167
Lewis acids, Lanthanide triflates 293
Lewis acids, water-tolerant 251
Lithium chloride 252 253
Lithium perchlorate 113 295 296
Lithium perchlorate-diethyl ether (LP-DE) 268Ч273 294
Lithium perchlorate-nitromethane 273Ч274 295
Lithium trifluoromethanesulfonimide 274Ч276 296
LUMO 22 23 24 36 107
Macrocycles 217 242
Maleic acid 149
Maleic anhydride 80 145 151 164 224 243
Maleic anhydride and 160 163
Maleic anhydride, 1,3-cyclohexadiene and 287
Maleic anhydride, 2-methoxy-1,3-butadiene and 117
Maleic anhydride, 2-vinylthiophene and 58
Maleic anhydride, anthracene and 99 157 160 163
Maleic anhydride, C-2 vinyl glical and 49
Maleic anhydride, cyclopentadiene and 14 164
Maleic anhydride, furan and 230 231 252
Maleic anhydride, isoprene and 286 288
Maleic anhydride, norboradiene and 18
Maleic anhydride, polycycles synthesis 43 81
Maleic anhydride, tropone and 226
Maleimide 116 117 230
Maleonitrile 8
Malic acid 258
Mannich conditions 290
Menthylacrylate 38 145
Metal ions, influence of 265
Methacrolein 133 147 259
Methanol 8 104 155 255
Methoxy carbonyl maleic anhydride 231
Methoxy cyclohexadiene 180
Methoxytrimethylsilane 6 128 129
Methyl acrylate 8 32 43 44 235 287
Methyl acrylate, 2-pyrone and 235
Methyl acrylate, cyclopentadiene and 117 147 178 255 280 282 283t 286
Methyl acrylate, ionic liquids and 280
Methyl acrylate, sulfinyldiene and 113
Methyl acrylate, zeolites and 148
Methyl cis-dihydrojasmonate 38
Methyl methacrylate 123
Methyl palustrate 243
Methyl propynoate 62
Methyl tanshinonate 195
Methyl vinyl ketone 144 156 170 255 266
Methyl vinyl ketone, 2-methoxy-3-thiophenylbutadiene 12
Methyl vinyl ketone, cyclopentadiene and 252
Methyl-5-aminofuroate 86
Methyl-trans-4-methoxy-2-oxo-3-butenoate 216
Methylalumoxane 134
Methylamine hydrochloride 264
Methylbenzoquinone 269
Methylglyoxylate 72 158
Methylpropiolate 117
Methylrhenium trioxide 266
Mevinolin 123
Micelles 283
Michael reactions 7 268
Microemulsion 281Ч283
MMX force field calculations 62
Monosaccharides 214
Morpholinoacrylonitrile 197
N,N-diethyltryptamine-N-oxide 63
N,N-dimethylacylamide 184
N-acryloyl oxazolidones 133 190
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте