|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Pippard A.B. — The Elements of Classical Thermodynamics |
|
|
 |
| Предметный указатель |
Absolute temperature, definition 35
Absolute temperature, establishment below  91 91
Absolute temperature, identical with gas scale 47 89
Absolute zero, unattainability, equipartition law 49
Absolute zero, unattainability, second law 48
Absolute zero, unattainability, third law 51
Additivity of thermodynamic functions 44
Adiabatic change 31
Adiabatic change, demagnetization 67
Adiabatic change, equation 61
Adiabatic change, surface 32
Adiabatic change, wall 5
Adiathermal process 16
Antiferromagnetism 140
Availability and useful work 101
Availability decrease in irreversible change 105
Availability of liquid drop 110 153
Availability of two-phase system 103
Availability, definition 100
Bernoulli's theorem 72
Boyle temperature 73
Boyle's law 12 47
Brownian motion 7 83
Caratheodory's statement of second law 30
Carnot cycle 33
Clapeyron's equation 53 115
Clapeyron's equation, analogues for superconductors 133
Clapeyron's equation, lambda transitions 143
Clapeyron's equation, second-order transitions 140
Clapeyron's equation, third-order transitions 142
Clausius's inequality 37 94
Clausius's inequality, statement of second law 29
Compressibility 61
Critical point, liquid-solid 122
Critical point, liquid-vapour 116
Curie temperature 91
Curie's law 67
Cyclical method 53
Demagnetization, adiabatic 67
Demagnotizing coefficient 66
Diathermal wall 5
Dieterici's equation 74
dimensions 43
Ehrenfest, classification of transitions 136
Ehrenfest, equations for second-order transitions 140
Enthalpy, conservation in stationary flow 70
Enthalpy, definition 43
Enthalpy, diagram for helium 76
Entropy of surface 86
Entropy, definition 37
Entropy, increase in Joule effect 69
Entropy, increase in Joule — Kelvin effect 72
Entropy, law of increase 95
Entropy, maximized in equilibrium 107
Entropy, related to equation of state, etc. 59
Eoetvoes's rule 86
Equation of state 10
Equilibrium, conditions for 104
Equilibrium, types of 6
exercises 160
Expansion coefficient 61
Extensive variables 44
Ferromagnetism, Weiss model 138
First law, statements 14 17
Fluctuations 7 83 96
Free energy (Helmholtz) minimized in equilibrium 107
Free energy (Helmholtz) of surface 85
Free energy (Helmholtz), definition 43
Free expansion see “Joule's experiment”
Friction 21
Gas thermometer correction 89
Gibbs function and phase equilibrium 108164
Gibbs function, definition 43
Gibbs function, g-surface 112
Gibbs function, magnetic 132
Gibbs function, minimized in equilibrium 107
Gibbs — Helmholtz equation 56
Heat, definition 16
Hotness and coldness 18
Indicator diagram 11
Intensive variables 44
Internal energy of surface 86
Internal energy, definition 15
Inversion curve 75
Inversion curve, temperature 73
Isentropic change, definition 31
Isothenn, critical 117
Isothenn, definition 11
| Johnson noise 83
Joule coefficient 57 58 69
Joule coefficient, effect 68
Joule — Kelvin coefficient 57 59 72
Joule — Kelvin coefficient, effect 69 89
Joule's experiment 21 42
Joule's experiment, law 47
Kelvin's statement of second law 30
Kelvin's statement of second law, vapour-pressure relation 111 154
Kirchhoff's Radiation Law 78
Lambda transitions 138
Lambda transitions in ammonium chloride 144
Lambda transitions in helium 126 143
Latent heat positive 114
Liquid drop, stability 153
Magneto-caloric effect 67
Maxwell's demon 99
Maxwell's demon, relations 45
Meissner — Ochsenfeld effect 131
Melting curve 113 122
Melting curve of helium 123
Noise, electrical 83
Order-disorder transformation in  -brass 138 -brass 138
Order-disorder transformation, Bragg — Williams model 138 151
Order-disorder transformation, degree and range of order 157
Order-disorder transformation, Ising model 140
Perfect gas, definition 47
Perfect gas, temperature scale 12 47
Phase diagram, 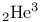 125 125
Phase diagram, 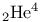 126 126
Phase diagram, iron 124
Phase diagram, simple substance 113
Phase equilibrium 112
Planck's radiation law 80
Prevost's theory of exchanges 80
Quasi-static change, definition 21
Quenching of disordered alloy 158
Radiation from sun 82
Radiation pressure 79
Radiation, black-body or cavity 77
Rayleigh — Jeans radiation law 83
Reversible process, definition 22
Second Jaw, statements 29 30
Specific heat,  -brass 138 -brass 138
Specific heat, helium 127
Specific heat, iron 141
Specific heat, manganous bromide 141
Specific heat, quartz 139
Specific heat, superconductor 134
Specific heats, principal always positive 18
Specific heats, principal difference 60
Specific heats, principal magnetic 65
Specific heats, principal ratio 61
Specific heats, principal variation with pressure, etc. 60
Stationary flow 70
Stefan — Boltzmann law 79 88
Sublimation curve 113
Superconductivity 129
Supercooling 114
Supercooling at second-order transition 152
Supercooling of superconductors 131 155
Supercooling of vapour 154
Surface tension 84
susceptibility 65
Temperature scale, absolute 35 47 91
Temperature scale, fixed points 88
Temperature scale, magnetic 91
Temperature scale, perfect gas 12 47
Temperature, definition 7
Temperature, empirical 10
Temperature, negative 52
Third law and surface tension 87
Third law, statement 51
Transitions, classification 137
Transitions, criticisms of theory 1 47
Transitions, Gorter's model 148
Transitions, lambda 126 142
Transitions, second-order 136 140
Transitions, third-order 140
Triple-point 113
Vapour-pressure curve 113
Virial coefficients 73
Work, reversible, elastic 23
Work, reversible, electric 27
Work, reversible, general 27
Work, reversible, magnetic 24
Work, reversible, surface tension 23
Zero, absolute 48
Zeroth law and converse, statements 9
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте




 -brass
-brass