|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rosser W.G.V. — An introduction to statistical physics |
|
|
 |
| Предметный указатель |
Absolute activity 295 317
Absolute temperature 68 69 70 157 231 235
Absolute zero 96 99 222
Accessible microstate 20 29 60
Accessible microstate, macroscopic system 48 51
Accessible microstate, number of 29 31 50 60
Adiabatic approximation 149 204 212 364
Adiabatic change 8 15 204 210 364
Adiabatic change of temperature 214
Adiabatic change, constancy of entropy 213
Adiabatic change, ideal boson gas 293
Adiabatic change, ideal fermion gas 293
Adiabatic change, statistical mechanics 210
Adiabatic demagnetisation 147 148
Adiabatic demagnetisation, experimental arrangement 150
Adiabatic partition 2 63
Adkins, C. J. 1 7 8 11 17 150
Andrew, E. R. 245
Andrews, F. C. 167
Atomic nucleus 305
Average value 336 340 347
Avogadro's constant 4 130 134 156 259
Band structure 305
Beta ( ) 51 67 68 71 241 ) 51 67 68 71 241
Betts, D. S. 100 266 279 288 289 290 295 299 315 316 323 327
Binding energy 193 194 196
Binding energy, diatomic molecule 193 196
Binomial distribution 45 46 338
Binomial distribution, average value 340
Binomial distribution, standard deviation 340
Binomial theorem 342
Black body radiation 246
Blackman, M. 261 267
Boltzmann constant 44 107 157 259 260
Boltzmann definition of entropy 101 205 206 207 280 281 365
Boltzmann distribution 99 105 108 203 206 233 253 295 317 321 325
Boltzmann distribution, isothermal atmosphere 129
Boltzmann distribution, thermodynamics via partition function 136
Bose Einstein condensation 319 321
Bose Einstein distribution function 276 277 314 319 321
Bose Einstein statistics 28 32 49 90 276 313
Bose temperature 315 317 320 328
Boson 28 313
Boson gas see "ideal boson gas"
Callen, H. B. 1 103 172
Canonical distribution 108
canonical ensemble 137 206
Carath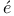 odory's principle 13 217 odory's principle 13 217
Carnot cycle 11 70 228 349
Carnot cycle, efficiency 11 232 351
Carnot cycle, statistical mechanics 228
Carnot's theorems 11 13 351
Celsius temperature scale 6 7
Centigrade temperature scale 6 7
Chandrasekhar limiting mass 311
Change of phase 185
Characteristic temperature 118 121
Characteristic temperature, atomic system 122
Characteristic temperature, dipole in a magnetic field 114
Characteristic temperature, harmonic oscillator 120
Characteristic temperature, ideal gas 121
Characteristic temperature, multilevel system 118
Characteristic temperature, nuclear system 122
Characteristic temperature, solid 122
Characteristic temperature, two level system 109
Chemical equilibrium 190
chemical potential 15 89 142 173 181 285 296
Chemical potential, classical limit 290
Chemical potential, classical thermodynamics 15
Chemical potential, Fermi energy 285
Chemical potential, Fermi level 301
Chemical potential, ideal boson gas 316
Chemical potential, ideal diatomic gas 167 ...cont.
Chemical potential, ideal fermion gas 288
Chemical potential, ideal monatomic gas 160
Chemical potential, multicomponent system 91 174 182
Chemical potential, partition function 143
Chemical potential, photon gas 324
Chemical potential, statistical mechanics 89
Chemical reaction 180 190
Chemical reaction, dissociation of a diatomic molecule 193
Chemical reaction, ideal gases 191
Classical limit 126
Classical limit, ideal boson gas 314 319
Classical limit, ideal diatomic gas 166
Classical limit, ideal fermion gas 290
Classical limit, ideal monatomic gas 155
Classical statistical mechanics 127
Clausius Clapeyron equation 7 189
Clausius statement of second law 10 74 83
Clausius' theorem 12 13 17
Closed system 3 34 38 61 63 86 105 107 108 271
Coexistence curve 188
Combinations 335
Configuration 29
Conservation of energy 61
Constraint 3
Cosmic background (3K) radiation 251 252
Courant, R. 368
Curie's law 114 116 117
de Broglie wavelength 297 372
Debye 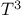 law 147 267 269 303 304 328 law 147 267 269 303 304 328
Debye angular frequency 263
Debye temperature 265 267 268
Debye theory of heat capacities 263
Degeneracy 24 31 117
Degenerate fermion gas 293
Density of states 91 92 94 246 282 289 300 356 367
Density of states, angular frequency 369
Density of states, elastic waves 370
Density of states, electromagnetic waves 370
Density of states, frequency 369
Density of states, particle in a box 356 370
Density of states, photon 371
Density of states, progressive waves 373
Density of states, thermal equilibrium 95 362
Density of states, wavelength 369
Diathermic partition 2 63
Diatomic molecule, binding energy 193 194 195
Diatomic molecule, chemical potential 167
Diatomic molecule, classical limit 166
Diatomic molecule, dissociation energy 195
Diatomic molecule, dissociation of 193
Diatomic molecule, electronic partition function 164
Diatomic molecule, heat capacity 166
Diatomic molecule, nuclear partition function 165
Diatomic molecule, rotation partition function 162
Diatomic molecule, total partition function 161 165
Diatomic molecule, translational partition function 161
Diatomic molecule, vibration partition function 164
Dissociation energy 195 196
Dugdale, J. S. 147
Dulong and Petit's law 259 262 265
Efficiency of a cycle 11 220 221 232 351
Ehrenfrest's principle 149 204 212 364
Einstein angular frequency 262 263
Einstein characteristic temperature 260 262
Einstein theory of heat capacity 260
Eisberg, R. M. 28
Empirical temperature 6
Energy eigenvalue 24 25 354 356
Energy eigenvalue, diagram 210
Energy eigenvalue, effect of changing volume 27 364
Energy eigenvalue, representative 210
Energy level 24
Energy of a thermodynamic system 93
Energy, ensemble average 43 137
Energy, fluctuations in 66 138
Ensemble average 41 43 44 109 137 138 140 274
Enthalpy 172 179 188
| entropy 12 13 19 44 51 68 71 94
Entropy, Boltzmann definition 101 205 206 207 280 281 365
Entropy, composite system 75 361 362 363
Entropy, density of states 94
Entropy, dependence on U, V and N 55
Entropy, extensive variable 77
Entropy, fluctuations in 80
Entropy, harmonic oscillator 142
Entropy, ideal boson gas 323 331
Entropy, ideal fermion gas 295 299
Entropy, ideal monatomic gas 157
Entropy, information theory 101
Entropy, mixing 158 170
Entropy, small system 140
Entropy, spin system 148
Entropy, variation with energy 19
Equilibrium constant 191 192 193 197 202
Equilibrium, closed system 62 185
Equilibrium, constant T and p 182 183 189 190
Equilibrium, constant T and V 174 176 186 191
Equipartition theorem 130
Exponential function 332
Extensive Variable 5 77
Fermi Dirac distribution function 274 276 283 285 287 289
Fermi Dirac statistics 28 32 90 274 282
Fermi energy 285 288 294 300 306 308
Fermi level 276 301
Fermi temperature 285 294 300 306 308
Fermion 31 282
Fermion gas see "ideal fermion gas"
Feynman, R. P. 23
First law of thermodynamics 7 72 222 350
Fluctuations in a macroscopic system 44
Fluctuations in a macroscopic system, energy 73 138 358
Free electron theory of metals 299
Free electron theory of metals, chemical potential 301
Free electron theory of metals, heat capacity 302
Free electron theory of metals, pressure 301
French, A. P. 28 162
Frenkel defect 199
Fundamental relation 103
Fundamental relation, ideal monatomic gas 157
Galvanometer suspension 135
Gas constant 156
Gasser, R. P. H. 133
Gaussian distribution 45 47 54 55 58 66 73 338 346 348 359 361 363
Gaussian Integrals 344
Gibbs free energy 90 103 172 180 190
Gibbs free energy, equilibrium at constant T and p 182 183 189 190
Gibbs Helmholtz equations 174 181
Gibbs paradox 32 159
Gopal, E. S. R. 163 165 262 266 267 292 304 305 315 317
Grand canonical distribution 271 273 325
grand canonical ensemble 207
Grand partition function 274 276 277
Grand partition function, ideal boson gas 322
Grand partition function, ideal fermion gas 297 298 299
Grand partition function, thermodynamics 278
Grand potential 278 279 280
Grand potential, ideal boson gas 322
Grand potential, ideal fermion gas 297 298
Grand potential, photon gas 325
Grand potential, thermodynamics 278
Gravitational potential energy 308
Griffiths, R. B. 98
Ground state 26
Guggenheim, E. A. 191
harmonic oscillator 25 120 138 247 252 260 264 269
Harmonic oscillator, characteristic temperature 120
Harmonic oscillator, classical limit 121 131
Harmonic oscillator, entropy 142
Harmonic oscillator, mean energy 121 138 141
Harmonic oscillator, partition function 120
Heat 8 203
heat capacity 18 19 71 259
Heat capacity, Debye's theory 263
Heat capacity, diatomic gas 166
Heat capacity, Einstein's theory 260
Heat capacity, electron gas 302
Heat capacity, ideal boson gas 317 323
Heat capacity, ideal fermion gas 291
Heat capacity, metal 269 302
Heat capacity, paramagnetic salt 147
Heat capacity, positive value 74 360
Heat capacity, spin system 147
Heat flow, direction of 10 16 40 74 83
Heat flow, direction of, negative temperatures 241
Heat reservoir 3
Heat, addition to a system 207 214
Helium, liquid 320 326
Helmholtz free energy 103 142 172 198 199 325
Helmholtz free energy, equilibrium at constant T and V 174 186 191
Helmholtz free energy, ideal gas 199
Helmholtz free energy, thermodynamic variables 174
Hilbert, D. 368
Hill, T. L. 167 192
Hook, J. R. 328
Huang, K. 299 302 323
Hydrogen atom 24
Ideal boson gas 313
Ideal boson gas, adiabatic expansion 293
Ideal boson gas, Bose temperature 315
Ideal boson gas, chemical potential 314 316 319
Ideal boson gas, classical limit 316
Ideal boson gas, energy spectrum 314
Ideal boson gas, entropy 323 331
Ideal boson gas, grand partition function 322
Ideal boson gas, grand potential 322
Ideal boson gas, heat capacity 317 323
Ideal boson gas, photons 324
Ideal boson gas, pressure 317 323
Ideal boson gas, thermodynamics 322
Ideal boson gas, total energy 317 323
Ideal boson gas, velocity distribution 314 315 321
Ideal cycle 217
Ideal fermion gas 282
Ideal fermion gas, adiabatic expansion 293
Ideal fermion gas, atomic nucleus 305
Ideal fermion gas, chemical potential 285 288 295
Ideal fermion gas, classical limit 290 295 296
Ideal fermion gas, degenerate 293
Ideal fermion gas, energy spectrum 287
Ideal fermion gas, entropy 295 299
Ideal fermion gas, grand partition function 297 298 299
Ideal fermion gas, grand potential 297 298 299
Ideal fermion gas, heat capacity 291
Ideal fermion gas, pressure 291 294
Ideal fermion gas, relativistic 310
Ideal fermion gas, thermodynamics 297
Ideal gas 5 156
Ideal gas thermometer 6 71 157
Ideal monatomic gas 156
Ideal monatomic gas, chemical potential 160
Ideal monatomic gas, entropy 157
Ideal monatomic gas, equation of 156
Ideal monatomic gas, fundamental relation 157
Ideal monatomic gas, Helmholtz free energy 199
Ideal monatomic gas, internal energy 156
Ideal monatomic gas, partition function 153 155
Indistinguishable particles 31
Indistinguishable particles, partition function 151
Information theory 100
Intensive variable 5
Irreversible process 16 233
Isolated system 38 61 78
Isothermal atmosphere 129
Isothermal process 8 225
Jauch, J. M. 254
Kelvin Planck statement of second law 10 205 215 217 349 352
Kelvin temperature, scale 6 11 69
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 )
) 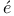 odory's principle
odory's principle 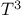 law
law