|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Gosser D.K. — Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms |
|
|
 |
| Предметный указатель |
Absolute energy scale 2
Acceptor number 32
Adsorption 43 97—99
Artemisinin 99—102
Benzoquinone reduction 7—10 11—12 79—80
Boiling point 32t
Born model of solvation 9—11
Capacitive current 57—59
Capacitive current in double step experiments 63
Capacitive current, simulation of 116—117
Catalysis of artemisinin reduction 100—102
Catalysis of glucose oxidase oxidation 87—88
Catalysis, Current follower 65—66
Catalysis, electron transfer chain 80—84
Catalysis, homogeneous redox 85—89
dielectric constant 18 32t 73
Diffusion 51—53
Diffusion, simulation of 105—108
Diffusion, visualization of 55
Dimensionless units 109—110
Donor number 32 73
Double layer 22—23
Electrochemical cell 5 28 30—31
Electrochemical cell, equivalent circuit for 56
Electrodes, luggin cappillary 30—31
Electrodes, reference 34—35 66—67
Electrodes, silver amalgam 33
Electrodes, working 33—34 66—67
Electrolytes 33
Electron transfer, Butler — Volmer equation 14 45 109
Electron transfer, free energy relationship, for 19—21
Electron transfer, inner sphere 16—17 19—21
Electron transfer, Marcus theory 17—19
Electron transfer, multielectron transfer 75—77 127.
Electron transfer, outer sphere 16—17
Entropy of reduction 11
Faradaic current 20
Fermi level energy 1—3 67
Ferricyanide reduction 1
Ferricyanide reduction, cyclic voltammetry 30
Ferricyanide reduction, simulation 126 128
Fick's law 51—52 105—107
Formal potential 7
Graphics 125
Heterogeneous rate constant 12 14 45 46
Hydrogen reference 3—4
| Inner sphere complex 16 19 74 101—102
IR drop 56—57
IR drop, simulation of 116—117
Koopman's theorem 9
Linear free energy relationship 12—15
Mean square diffusion 38
Melting point 32t
Methylcobalamin reduction 30 47 139—146
Methylcobalamin reduction, simulation 126 129
Microelectrodes 59
Microelectrodes, used to determine electron number 59—69
Nernst equation 3—4 45
Nitric oxide 92—94
Nitrobenzoic acid 90—92
Nitrogen dioxide 72—75
Nitrogen dioxide, simulation 126—127 130—131
Operational amplifiers 64—66
Peak current, reversible one electron transfer 43 44
Peak current, shifted ratio 43
Peak potential, Reversible one electron transfer 42 44
Potential of zero charge 22 24
Potential step chronoamperometry 61—63
Potential step chronoamperometry of methylcobalamin 140—141
Potential step chronoamperometry of nitrogen dioxide 72—73
Potential, electrical 3 5
Potential, electrode 3—5 6—7 27
Potential, window 32 35
Potentiostat 64—68
Protonations 77—80 133
Reduction potential 1—6
Reduction potential for 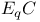 mechanism 139—146 mechanism 139—146
Reduction potential for 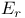 mechanism 43 mechanism 43
Reduction potential for 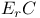 mechanism 49 mechanism 49
Reversibility 36 45—46 49—50 144—145
Scan rate 28
Scan rate and diffusion 51—52
Solvation 2 8 9—11
Solvatochromatic 321
Solvents 31—32
Solvents, properties 32t
Tempo oxidation 10
Transfer coefficient 14—16 47 145—146
Tricarbonyl(mesitylene)tungsten oxidation 95—97
Voltage amplifier 65—66
Voltage follower 65
work function 1
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



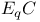 mechanism
mechanism 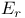 mechanism
mechanism 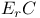 mechanism
mechanism