|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rushbrooke G.S. — Introduction to Statistical Mechanics |
|
|
 |
| Предметный указатель |
Adsorption with dissociation 217
Adsorption, Fowler's isotherm 310
Adsorption, Langmuir's isotherm 214 217 249 286 288 317
Anharmonioity 115
Band spectra 114
Boltzmann's constant 7 12 49 124
Boltzmann's H-theorem 314
Bragg and Williams approximation 294 309
Chemical potential of condensed phase 35
Chemical potential of monatomic gas 49
Chemical potential of perfeot gas 50 190
Chemical potential, and law of mass action 187
Classical statistics, condition for 46
Conversion factors 121
Critical adsorption 310
Critical mixing temperature 296 306 307
Darwin — Fowler method 312
Debye, specific heat of solid 34 140 147
Definitions, absolute activity 196
Definitions, assembly 4
Definitions, calorimetric entropy 138
Definitions, characteristic temperature 93
Definitions, classical statistics 46
Definitions, complexion 11
Definitions, component 5
Definitions, degenerate gas 46
Definitions, distribution numbers 17
Definitions, Einstein model 198
Definitions, ensemble 236
Definitions, equilibrium constant 186
Definitions, equilibrium constant, set of distribution numbers 22
Definitions, grand partition function 274
Definitions, independent systems 15
Definitions, internal distribution function 74
Definitions, localized systems 15
Definitions, metastable equilibrium 109
Definitions, non-localized systems 36
Definitions, partition function, assembly 237
Definitions, partition function, system 24 40 59
Definitions, perfect solution 226
Definitions, phase integral 60
Definitions, phase integral, space 56
Definitions, practical entropy 144
Definitions, quantal statistics 46
Definitions, regular solution 291
Definitions, representative point 56
Definitions, restrictive condition 17
Definitions, species 5
Definitions, spectroscopic entropy 168
Definitions, symmetry factor 88
Definitions, system 4
Definitions, thermodynamic method (for polyphase assemblies) 205
Definitions, weight factor 25
Dieleotric constant of gases 165
Dimerization, in condensed phase 214 286
Dimerization, in solution 230 286
Dipole moments 167
Dissociative equilibrium, gas phase, A+A = 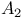 194 284 194 284
Dissociative equilibrium, gas phase, A+B = AB 178 282
Dissociative equilibrium, gas phase, AB+CD = AC+BD 197
Dissociative equilibrium, gas phase, general 196 284
Doppler broadening 78
Duhem — Margules equation 230 234 310 327
Dulong and Petit, law of 33 65
Einstein model 198 214 218 224 279 284 289 315
Einstein — Bose statistics 45 63 286
Einstein, specific heat of solid 30
Energy, classical law 66 70
Entropy of assembly 249
Entropy of mixing, of gases 176 186
Entropy of perfect solutions 229 234
Entropy of polyatomic rigid molecules 135
Entropy, and disorder 8
Entropy, rotational, of diatomic molecule 98 154
Entropy, rotational, of rigid molecule 153 154
Entropy, translational 48
Entropy, vibrational 35 97
Eulerian angles 131 157
Fermi — Dirac statistics 45 63 286
Fluctuation effects 2 28
Gibbs paradox 177
Gibbs — Helmholtz formula 32 237 302 318
Grand partition function for [1]-regular solution 299
| Grand partition function, defined 274
Grand partition function, references 332
Grand partition function, table of 286
Guggenheim's equation of quasi-chemical equilibrium 300 310
Heisenberg's uncertainty relation 55
Hindered rotation 150
Imperfect gas 46 250 281
Imperfect mixture 270
Intensity of spectral lines 116
Internal distribution functions 9 81
Internal distribution functions, linear rotator 117
Internal distribution functions, Maxwell — Boltzmann law 78
Internal distribution functions, ortho-para-hydrogen ratio 103
Internal distribution functions, polar gases in external field 156
Internal distribution functions, [1]-harmonic oscillators, classical 76
Internal distribution functions, [1]-harmonic oscillators, quantal 75
Internal distribution functions, [2]- and [3]-harmonic oscillators 83
Ising model 296
Isotopes 144
Lagrange's undetermined multipliers 19 207 273 324
Langevin function 162
Law of mass action, condensed phase 217
Law of mass action, gas phase 185 282
Maxwell — Boltzmann law 78 83
Metastable equilibrium 109 141
Multinomial theorem 243 322
Nernst's law 136 143
Non-rigid molecules 149 309
Nuclear spin 101 143
Partition function for assembly, defined 237
Partition function for assembly, imperfect gas 265
Partition function for assembly, independent localized systems 244
Partition function for assembly, independent non-localized systems 246 252
Partition function for assembly, perfect solution 289
Partition function for assembly, regular solution 291
Partition function, defined 24 40 59
Partition function, diatomic gas, classical 89
Partition function, diatomic gas, classical, quantal 93
Partition function, electronic (in molecule) 96
Partition function, factorization property of 80 248
Partition function, monatomic gas 127
Partition function, orientational-rotational 157
Partition function, rotation of diatomic molecule, classical 88
Partition function, rotation of diatomic molecule, classical, quantal 91
Partition function, rotation of diatomic molecule, deuterium 110
Partition function, rotation of diatomic molecule, hydrogen 102
Partition function, rotation of diatomic molecule, rigid moleoule 130
Partition function, translational, classical treatment 63
Partition function, translational, numerical value 53
Partition function, translational, quantal treatment 43
Partition function, [1]-harmonic oscillator, classical 65
Partition function, [1]-harmonic oscillator, classical, quantal 32 63
Partition function, [1]-rotator 152 154
Partition function, [2]-harmonic oscillator 35
Partition function, [3]-harmonic oscillator 32
Perfect gas 51
Phase separation 296 306 327
Physioal constants 121 330
Polarizability 165
Polymers 309
Raoult's law 227 282 287
Rotational levels and selection rules 113
Sackur — Tetrode equation 48 136 146 154 176
Specific heat, diatomic gas, classical 89
Specific heat, diatomic gas, classical, quantal 92 94
Specific heat, electronic (in molecules) 95
Specific heat, equilibrium hydrogen 105
Specific heat, hindered rotation 152
Specific heat, hydrogen 90
Specific heat, monatomic gas 48
Specific heat, ortho- and para-hydrogen 99
Specific heat, polyatomic rigid molecules 134
Specific heat, solid 30
Specific heat, vibrational 97
Stirling's formula 20 28 245 310 326
Temperature-dependent energy levels 316
Translational energy levels 40
Van der Waals' equation 268
Vibrational levels and selection rules 113 115
Vibrations of polyatomic molecule 128
Virial expansion 259 267 270
Virial expansion for mixture 271
Volume of condensed phase, effect of 209 284
Zero-point energy 31 76
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте