Авторизация
Поиск по указателям
Harrison W.A. — Elementary electronic structure
Обсудите книгу на научном форуме Нашли опечатку?
Название: Elementary electronic structureАвтор: Harrison W.A. Аннотация: This text presents an account of analytic electronic structure, to be distinguished from computational electronic structure. Both are based upon a one-electron approximation, local-density theory, and the determination of the quantum-mechanical electronic states. They both seek to predict the properties of the resulting solids, or molecules on the basis of these states.In the computational mode, the minimum number of approximations are used, and numerical solutions are sought. Here we seek instead to focus on the most important aspects of the solution, making what approximations are necessary in order to proceed analytically and obtain formulae for the properties. This reducing of the problem to basics is almost always less accurate that the computational solution, but has the advantage that it displays the dependence of any property on the parameters of the system. It gives us an understanding of the property in a sense that a numerical solution, or a direct measurement, cannot.
Язык: Рубрика: Физика /Серия: Библиотека «Математическое просвещение» Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Год издания: 1999Количество страниц: 819Добавлена в каталог: 19.03.2006Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
230 11—16 51pr 52pr 52pr see “Silicon dioxide” A15 structure 592 Abruptness of repulsion 89 117 Absorbtion threshold 216ff 221 Absorption of light see “Optical absorption” Accommodation 556 Acoustic vibrational modes 124 (see also “Vibrational frequencies”) Actinides see also “f-shell metals” Actinides, cohesive energy 618 619 Actinides, compounds 682ff Actinides, crystal structure 620 624 Actinides, volume-dependent energy 619ff Adsorption, atoms on semiconductors 749ff Aliased interactions 724 Alkali halides see “Ionic crystals” Alkali halides, Susceptibility 382ff Alkali metals, high pressure transition 576 (see also “Simple metals”) Alloys, energy bands 304 Alloys, semiconductors 294ff Alloys, simple metals 513ff Alloys, structure 298 Alloys, transition metals 578ff Alumina, 430 Americium 637 (see also “ f-shell metals”) Anderson localization 625ff Angular forces 104 131ff Angular forces, hydrides 782 Angular forces, metals 496 Angular forces, silicon dioxide 411ff Antibonding bands 183ff Antibonding states, 13 Antibonding states, semiconductors 63 Antiferromagnetism, chromium 552 Antiferromagnetism, cuprates 708ff Antiferromagnetism, f-shell metals 638 Antiferromagnetism, transition metals in general 590 Antisite defect 312 313 Antisymmetry of electron states 450 Atomic electron states 5 Atomic electron states, core states 8 Atomic electron states, term values, plot 7 Atomic electron states, term values, table for d-states 645 Atomic electron states, term values, table for f-states 683 Atomic electron states, term values, table for s, p-states 9 Atomic Sphere Approximation 554ff 565 Atomic sphere radius, simple metals, table 436 Atomic sphere radius, transition metals, table 539 Atomic Surface Method 531 556ff 672ff Auger processes 321 Average lattice 298 304 Axial ratio, hep structures 437 488 586 Axial ratio, hep structures, table 438 Baldereschi points 348ff Ball-and-stick model for semiconductors 137 138 Band gap see “Energy gap” Band iine-ups at interfaces 741ff Band structure see “Energy band” Band width, Atomic Surface Method 558 Band width, d-bands 549 560 Band width, f-shell metals 603 Band width, narrowing in cuprates 706 Band width, second moment 560ff Band-structure energy, simple metals 483 Bechstedt form for overlap repulsion 67 68 80 90 Beta cristobalite 400 422 Beta-eucryptite 430 Binding energy of solids see “Cohesive energy” Bloch sums 18 56 345 Body-centered-cubic structure 183 437 Bom Model, ionic crystals 330 339ff 367 716 Bom-Oppenheimer Approximation 207 Bond energy see “Cohesive energy” Bond length, 758 Bond length, boron 760 Bond length, dependence upon coordination 99 100 Bond length, effect of metallization 85 Bond length, graphite, prediction for 106pr Bond length, inert-gas solids 768 Bond length, ionic crystals 361ff Bond length, rare-earth nitrides 682 Bond length, simple metals see “Nearest-neighbor distance” Bond length, table, ionic crystals 330 Bond length, table, semiconductors 60 211 216 Bond length, transition metals see “Atomic sphere radius” Bond length, transition-metal compounds 660 661 Bond length, trends in semiconductors 85ff Bond order, transition-metal alloys 582 Bond-charge model 132 Bond-Orbital Approximation, diatomic molecule 28 Bond-Orbital Approximation, mathematics of 74 Bond-Orbital Approximation, semiconductors 70ff Bond-Orbital Approximation, silicon dioxide 407 411ff Bond-Orbital Approximation, support for, from moments 98 Bonding states, bonding bands 183ff Bonding states, dipoles 143 144 Bonding states, floating 777 Bonding states, graphite 106pr Bonding states, in 13 Bonding states, metallization 75ff Bonding states, multicenter 754ff Bonding states, pseudopotential theory 508ff Bonding states, three-center (delta) 759 Bonding unit 308 Bonding unit, boron 759 Bonding unit, impurity states 307ff Bonding unit, multicenter bonds 754ff Bonding unit, silicon dioxide 400 401 Boron 759ff Bowing of energy bands 305 Bragg reflection 465 Bravais lattice 56 Brillouin Zone, crystals 180ff Brillouin Zone, cuprates 706 Brillouin Zone, extended and reduced 472 Brillouin Zone, one dimension 18 Bucky balls 773—775 Bulk modulus, 758 Bulk modulus, boron 760 Bulk modulus, fiuorite 352pr Bulk modulus, ionic crystals 351 352pr 363ff Bulk modulus, rare earths 617 618 Bulk modulus, ruthenium dioxide 688 Bulk modulus, semiconductors 87 108ff Bulk modulus, silicon dioxide 416 Bulk modulus, simple metals 443 494 496 Bulk modulus, transition metals 574 575 Bulk modulus, transition-metal compounds 667 c/a ratio, hep structures 437 488 586 c/a ratio, hep structures, table 438 Calcium see “Simple metals” Calcium fluoride see “Fiuorite” Calcium, structure determination 578 Carbyne, elasticity, metallization 141pr Carbyne, energy bands 213pr Carbyne, vibration spectrum 134ff Cauchy relations 367 375 496 497ff Cellular method 554 Central-hydride molecules 781ff Cerium 603 608 611ff 622 635ff Cerium, nitride 681 Cesium-chloride structure 329 Cesium-chloride structure, overlap repulsion in 360 Charge-density wave 708 Chemical grip 368 700 Chromium, electronic structure 550ff Cohesive energy, 758 Cohesive energy, actinide compounds 687 Cohesive energy, actinides 618 619 Cohesive energy, boron 760 Cohesive energy, Coulomb corrections, semiconductors 82ff Cohesive energy, dependence upon polarity 82 Cohesive energy, elemental semiconductor, formula 80 Cohesive energy, elements, table 21 Cohesive energy, inert-gas solids 768 Cohesive energy, ionic crystals 336 363 Cohesive energy, ionic crystals, fiuorite 352pr 353pr Cohesive energy, ionic crystals, table, divalent 338 Cohesive energy, ionic crystals, table, monovalent 337 Cohesive energy, nitrogen molecule 27 Cohesive energy, one-dimensional chain 20 Cohesive energy, perovskites 694 Cohesive energy, rare earths 614ff Cohesive energy, semiconductors 70ff Cohesive energy, semiconductors, table 80 Cohesive energy, silicon dioxide 406ff Cohesive energy, simple metals 440ff Cohesive energy, transition metals 569ff Cohesive energy, transition-metal compounds 647 667 678pr Complementarity 556 Compounds, f-shell-metals 680ff (see also “Ionic crystals” “Semiconductors” Conduction band 64 Conduction band, ordering of levels at r 176 177 Configuration interaction 83 Core radius 39 (see also “Empty-core pseudopotentials”) Core states 8 Correlated states 84 599 Correlated states, criteria for 601 631ff Correlated states, cuprates 709ff Correlated states, formation of local moments 625ff Correlated states, role in lattice spacing 622 Correlation energy 83 207 Correlation energy, band narrowing in cuprates 707 Correlation energy, effects on magnetism 591 Correlation energy, f-shell and transition metals 593ff Correlation energy, free-electron gas 451 Correlation energy, photothresholds and work functions 727 Correlation energy, transition-metal compounds 668 Correlation energy, Van-der-Waals interaction 765ff 768 Coulomb effects see also “ Madelung energy” Coulomb effects, band gaps 206ff 326 341 423 Coulomb effects, band narrowing in cuprates 707 Coulomb effects, cohesion of semiconductors 82ff Coulomb effects, d-state shifts in transition metals 561ff Coulomb effects, d-state shifts in transition metals, from atomic calculations 561 Coulomb effects, impurities in semiconductors 745 Coulomb effects, in compounds 659ff 691 695 705 Coulomb effects, ionic crystals, bonding 372ff Coulomb effects, ionic crystals, elastic constants 375ff Coulomb effects, Madelung potential 82 Coulomb effects, photothresholds and work functions 50 727 Coulomb effects, surfaces 716 Coulomb repulsion, U 10 Coulomb repulsion, U, d-states 46 561 Coulomb repulsion, U, d-states, in f-shell metals 610ff Coulomb repulsion, U, d-states, table 539 Coulomb repulsion, U, f-states 607ff Coulomb repulsion, U, sp-atoms, table 9 Coulomb repulsion, U, total energy in semiconductors 69 Coupling between electronic states see “Matrix elements” Covalency 63 Covalent energy, 29 Covalent energy, f-shell-metal compounds 680 Covalent energy, from second moment, 98 Covalent energy, ionic crystals 344 352pr 359 Covalent energy, perovskites 695 Covalent energy, pseudopotentials, related to 512 513 Covalent energy, silicon dioxide 404 Covalent energy, sp-hybrids, defined 59ff Covalent energy, sp-hybrids, defined, table 60 Covalent energy, transition-metal compounds 657 Crystal structure, actinides 620 624 Crystal structure, alkali metals under pressure 576 Crystal structure, beta cristobalite 400 Crystal structure, body-centered-cubic 437 Crystal structure, cesium chloride 329 Crystal structure, cuprates 706 Crystal structure, density of states role 577 57 Crystal structure, face-centered-cubic 54ff 434ff Crystal structure, fluorite ( 351 Crystal structure, graphite 105 Crystal structure, hexagonal close-packed 437 Crystal structure, ionic crystals 329 Crystal structure, packing fractions 481pr Crystal structure, perovskite 693 Crystal structure, prediction for graphite 104 Crystal structure, pyrite 430 Crystal structure, rare earths 618 Crystal structure, rock-salt 329 Crystal structure, rutile 688 Crystal structure, S, Se, and Te 752 Crystal structure, semiconductors 53ff Crystal structure, semiconductors, prediction 99ff Crystal structure, silicon dioxide 400ff Crystal structure, simple metals 435ff Crystal structure, simple metals, prediction 488 Crystal structure, transition metals 576ff Crystal structure, wurtzite 57 Crystal structure, zincblende 53ff Crystallographic directions 55 Cuprates 49 704ff Curie temperature 590 Current operator and continuity 739 d-states 6 534ff d-states, Coulomb shifts in compounds 659ff 705 d-states, Coulomb shifts in transition metals 561 d-states, coupling between 542 d-states, coupling between, Slater — Koster tables 546 d-states, energy bands 45 545ff 592pr d-states, energy bands, 592pr d-states, energy bands, band widths, table 539 d-states, f-shell metals 610ff d-states, localization of 43 530 d-states, overlap repulsion 568 569 582 d-states, radius, 43 531 d-states, radius, 537 d-states, radius, 558 d-states, radius, 539 674 d-states, self-consistent shifts of 45 561 659ff 705 d-states, semiconductors, role in 672ff Dangling hybrids, or bonds 314 Debye — Huckel screening 289 Debye — Waller factor 478 Deep levels 309ff Defects, antisites 312 313 Defects, interstitials in semiconductors 317 Defects, vacancies in semiconductors 314ff Deformation potentials 254ff 270 292pr Deformation potentials, strain-layer superlattices 745ff Dehybridization energy, silicon dioxide 413 Density of states, chromium nitride 651 Density of states, misleading for some problems 704 Density of states, semiconductors 245ff Density of states, transition metals 552ff Density of states, wavenumber space 182 Density-functional theory 5 664ff Diagonalization of matrices 18 73ff 95 Diamagnetism, semiconductors 159 dielectric constant see “Dielectric susceptibility” Dielectric constant, defined 147 Dielectric constant, static 165ff Dielectric constant, table for semiconductors 211 Dielectric function 289 469 Dielectric function, Lindhard or Hartree 462 485 Dielectric properties, ionic crystals 379ff Dielectric properties, semiconductors 143ff Dielectric susceptibility, inert-gas solids 764ff Dielectric susceptibility, ionic crystals, local-field effects 387ff
Реклама
 |
|
О проекте
|
|
О проекте



 method
method 
 states
states 
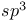 -hybrids
-hybrids 
 )
)