|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Chivers T. — A Guide to Chalcogen-Nitrogen Chemistry |
|
|
 |
| Предметный указатель |
 ring 267 ring 267
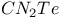 ring 213 ring 213
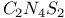 rings 22 rings 22
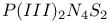 ring 267 ring 267
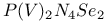 ring 270 ring 270
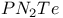 ring 26 267 ring 26 267
![$[Et_4P_2N_4S_2]^{2+}$](/math_tex/bf8b70dd65d241255cf0b30638718a6882.gif) dication 263 dication 263
![$[Ph_4P_2N_4S_2]^{2-}$](/math_tex/024b8b850e6208ea87906624e67dc99582.gif) dianion 263—265 dianion 263—265
![$[Te_2S_2N_4]^{2+}$](/math_tex/62da27831db6c6c9264594f513dc304e82.gif) dication 155 dication 155
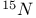 -enriched -enriched 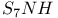 23 23
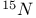 -enriched -enriched ![$[S_3N]^-$](/math_tex/43084759b8c808f05b736141d660cbdb82.gif) 100 100
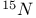 -enrichment, -enrichment, 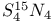 47 47
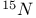 -labelled S-N compounds 19 -labelled S-N compounds 19
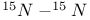 coupling 34 coupling 34
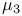 -Nitrido 155 -Nitrido 155
1,2,3,4-Trithiazolium radical cations 224
1,2,3,5-Dithiadiazolium cation 214—216
1,2,3,5-Dithiadiazolyl radicals 67 217—220
1,2,3,5-Dithiadiazolyls 67
1,2,3-Dithiazolium 224
1,2,3-Dithiazolyls 226
1,2,3-Selenadiazole 232
1,2,5-Selenadiazole 228 231
1,2,5-Telluradiazole 229—231
1,2,5-Thiadiazoles 88 228 230 231
1,2-Selenazine 182
1,3,2,4-Dithiadiazolium cation 221
1,3,2,4-Dithiadiazolyl radicals 222
1,3,2-Dithiazolium 226
1,3,2-Dithiazolyls 67 226
1,3-Metallotropic shift 265
1,3-Nitrogen shifts 34 74 75 254
2-Selenobenzylamine 298
5-Oxo-1,3,2,4-dithiadiazole, 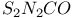 223 223
Acyclic cation, ![$[N(SCl)_2]^+$](/math_tex/42962e7fd0fc3863d18923c3ece148ed82.gif) 147 147
Acyclic cation, ![$[N(SeCl)_2]^$](/math_tex/c7977ff62ebeb7989e91d96d2e1fb1c382.gif) 147 147
Acyclic cation, ![$[N(SeCl_2)_2]^+$](/math_tex/a70e4de54152688c93a149fddf433d1982.gif) 147 147
Allylic amination 185
Anion, ![$[NSCl_2]^-$](/math_tex/eee3c8fdab3daa78c8a7c62ddee8bf4382.gif) 143 143
Anion, ![$[NSF_2]^-$](/math_tex/8e7ee00b2afdcd5c6f2897ea0e51a32382.gif) 142 142
Anion, ![$[NSF_2]^-$](/math_tex/8e7ee00b2afdcd5c6f2897ea0e51a32382.gif) , complex 132 , complex 132
Anion, ![$[N_3Se_3O_6]^{3-}$](/math_tex/bcf484a2860b848d19aaacc9ac1faf4982.gif) 175 175
Anion, ![$[N_3S_3O_3F_4]^-$](/math_tex/c8bae50383345587da7dce4b6822130582.gif) 154 154
Anion, ![$[N_3S_3O_6]^{3-}$](/math_tex/c55f2a989216821f8d70ab43832f4ea682.gif) 175 175
Anion, ![$[Se_2N_2]^{2-}$](/math_tex/4722f190e046fc6beb3c019a3ec5fe7482.gif) , complexes 128 , complexes 128
Anion, ![$[Se_3N]^-$](/math_tex/f328b2547bcbe13cf15fb9c9452bde7c82.gif) , complexes 129 , complexes 129
Anion, ![$[SNO]^-$](/math_tex/18cf5d81a268a47634a1e72312df83b782.gif) see “Thionitrite” see “Thionitrite”
Anion, ![$[SN]^-$](/math_tex/5695f9f0e634df4e3f993965dfe7246082.gif) , complexes 131 , complexes 131
Anion, ![$[SN_2]^{2-}$](/math_tex/2e96f88a782861b46d5636637485d98d82.gif) 98 99 98 99
Anion, ![$[SN_2]^{2-}$](/math_tex/2e96f88a782861b46d5636637485d98d82.gif) , complexes 136 , complexes 136
Anion, ![$[SO_2N_3]^-$](/math_tex/085606397b70df665422dbfa88eb4ef382.gif) 165 165
Anion, ![$[SO_3N_3]^-$](/math_tex/2c4d4a56d241ae7357e218d7170e8c4382.gif) 165 165
Anion, ![$[SSNO]^-$](/math_tex/6ea53a824e79f2b141758baef901bebd82.gif) see “Perthionitrite” see “Perthionitrite”
Anion, ![$[SSNSS]^-$](/math_tex/a3688d9feecc97f9e108df8da42235ae82.gif) 100 100
Anion, ![$[SSNS]^-$](/math_tex/bf8929925b1f768fa6d488aa136c123b82.gif) 100 100
Anion, ![$[SSNS]^-$](/math_tex/bf8929925b1f768fa6d488aa136c123b82.gif) , complexes 128 , complexes 128
Anion, ![$[S_2N_2H]^-$](/math_tex/e3b0b676f2ec749b4da407f8f512c55982.gif) 101 101
Anion, ![$[S_2N_2H]^-$](/math_tex/e3b0b676f2ec749b4da407f8f512c55982.gif) , complexes 127 , complexes 127
Anion, ![$[S_2N_2]^{2-}$](/math_tex/5a86942a3d778e334492d3e4d349a97c82.gif) , complexes 128 , complexes 128
Anion, ![$[S_2N_3]^{3-}$](/math_tex/2d486d244b4a8b7ba541f99f1f14547582.gif) , complexes 130 , complexes 130
Anion, ![$[S_3N_3O]^-$](/math_tex/da70cba52f9eebe36c01a823748ed50182.gif) 174 174
Anion, ![$[S_3N_3O_2]^-$](/math_tex/df7dbb26a9bb08a10746151c8505f0c082.gif) 174 174
Anion, ![$[S_3N_3O_4]^-$](/math_tex/754a9f83c08ca6a37a2938da5337f64582.gif) 174 174
Anion, ![$[S_3N_3]^-$](/math_tex/5ed988a3ae98a787c6d2d605b6b9867682.gif) 102 102
Anion, ![$[S_3N_4]^{2-}$](/math_tex/c11da2e47a1e6473ebf49ec016731c8782.gif) , complexes 130 , complexes 130
Anion, ![$[S_4N_3]^-$](/math_tex/2c6b255e5ccc0d63a370006dcca3020a82.gif) , complexes 130 , complexes 130
Anion, ![$[S_4N_4]^{2-}$](/math_tex/57e223b2246255f70d395b9f9f85284082.gif) , complexes 130 , complexes 130
Anion, ![$[S_4N_5O]^-$](/math_tex/675a3a4f46e9c22b881eddeed075a97482.gif) 175 175
Anion, ![$[S_4N_5O_2]^-$](/math_tex/11179ff73f528ddc9259a4548c6c612482.gif) 175 175
Anion, ![$[S_4N_5]^-$](/math_tex/954f2c1d3cb46b072ffc9eec6efa94c582.gif) 103 103
Anomeric effect 150 245
Antiaromatic systems 61
Antimony derivatives 266
Aromaticity 58 60
Arsenic derivative, 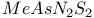 265 265
Arylselenium azides 23 306
Atacticity 290
Azadisulfite dianion 171
Azasulfite anions 171
Benzenesulfinyl azide, 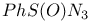 202 202
Benzo-1,2,3-thiadiazole 231
Benzo-1,2,5-selenadiazole 228
Benzo-1,2,5-telluradiazoles 229
Benzo-1,2,5-thiadiazole 231
Benzo-1,3,2-dithiazolyl 201
Benzodithiadiazine 245 246
Benzotrithiadiazepine 247
Bicyclic compound, 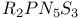 261 261
Bicyclic ring, 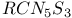 253 254 253 254
Bis(sulfinylamino)selane, 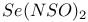 166 166
Bis(sulfinylamino)sulfane, 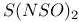 166 166
Bis(sulfinylamino)tellane, 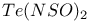 166 166
Bistability 227
Chalcogen-nitrogen bonds, formation 18—28
Chiral organoselenyl halides 306
Conducting polymers 280
Conductivity, 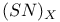 56 56
Covalent radii 2
Cyanuric-sulfanuric system 243 251
Cyanuric-thiazyl system 242
Cyclic chalcogen imides 3 4 111—119
Cyclic selenium imides 117—119
Cyclic sulfur imides 112—117
Cyclic tellurium imide, 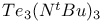 117 117
Cycloaddition 69—71
Cycloaddition, NSF 142
Cyclocondensation 18 22 24 262
Cyclometallathiazenes 125—129 167 265
Cyclopentathiazyl cation, ![$[S_5N_5]^+$](/math_tex/d4728a1e0b39682b9501cee433a9eec282.gif) 97 97
Cyclophosphadithiatriazines, 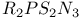 260 261 260 261
Cyclotetrathiazene dioxide, 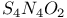 174 174
Cyclotetrathiazyl dication, ![$[S_4N_4]^{2+}$](/math_tex/b51e4240b524e660ef57c21590160d1f82.gif) 96 96
Cyclotetrathiazyl fluoride,  152 152
Cyclotrithiazyl cation, ![$[S_3N_3]^+$](/math_tex/0391c895503cc8b2eea144e466624f1e82.gif) 95 95
Cyclotrithiazyl chloride, 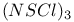 149—152 149—152
Cyclotrithiazyl fluoride, 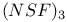 149—150 149—150
Diaminopolyselanes,  199 199
Diaminoselanes, 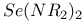 199 199
Diaminosulfanes, 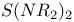 199 199
Diaminotellanes, 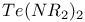 31 199 31 199
Diazasulfite anions 170
Dibenzenesulfenamino radical, ![$[(PhS)_2N]^{\bullet}$](/math_tex/5d647c987295f0ba036f125e575d2b2382.gif) 202 202
Diels — Alder 182 248
Diffraction techniques 33—36
Dihalocyclotetrathiazenes 152
Dinitrogen sulfide, 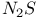 82 82
Diphosphadithiatetrazocines 261
Diradical 223 224
Diselenadiazolyl radicals 217
Diselenium dinitride, 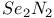 83 83
Disproportionation energies 218 226
Disulfur dinitride, 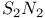 83 84 83 84
Ditelluradiazolyls, ![$[RCTe_2N_2]^{\bullet}$](/math_tex/cf4d88039267db0fc500fa177733fa3e82.gif) 31 31
Dithiadiazenes, ArNSNSAr' 284
Dithianitronium cation, ![$[S_2N]^+$](/math_tex/7cd31f1f66d6a6b29719c23597c84ba682.gif) 92 92
Dithiatetrazocines 64 72 248—250
Dithiatriazines 66 221 243
Dithiatriazyl cation, ![$[S_2N_3]^+$](/math_tex/e0c5afd5eb7f943a5333245922a72e5582.gif) 95 95
Doping, halogens 281
Eight-membered rings, 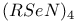 36 36
Electrochemical reduction, 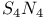 41 74 41 74
Electrochemical reduction, ![$[S_5N_5]^+$](/math_tex/d4728a1e0b39682b9501cee433a9eec282.gif) 43 43
Electrochemical studies 41 42 218—220
Electron density studies 187 197
Electron diffraction 31 32 144 145 187 245
Electron-counting procedure 5
Electron-rich aromatics 53 58
Electronegativities 1 166
Electronic structures 53—68
EPR spectroscopy 37—41 222
| Ferromagnet 217
Fluxional process 37 75 254
Fremy's salt 37
Frontier orbital theory 69—73
Glass transition temperature (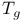 ) 286 288 ) 286 288
Glutathione peroxidase 302
Gunpowder reaction 164
Halogen exchange 133 150 152
Heat of formation,  85 85
Heterocyclothiazenes 259—273
Heterocyclothiazenes, bonding 60
Hueckel rule 5 53 58 91
Hund's rule 60
Hypervalent 299
Imidoselenium(II) dihalides 118—157
Imidoselenium(IV) dihalides 156
Imidosulfur(IV) dihalides 156
Imidotellurium dihalides 156
Imidotelluroxane 169
Infrared spectroscopy 46
Intermolecular 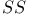 interactions 62 interactions 62
Intermolecular 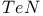 contacts 31 contacts 31
Intermolecular  interactions 7 8 interactions 7 8
Intermolecular association 66—68
Intramolecular 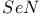 interaction 299 302 interaction 299 302
Intramolecular  interactions 6 7 65 interactions 6 7 65
Intramolecular chalcogen-chalcogen interactions 63—65
Intramolecular chalcogen-nitrogen interactions 294—306
Ionization potential, NS 81
Ionization potentials, 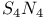 43 43
Ipsocentric ring current 59
Isolobal 60 73 240 260
Isotactic 290
Isotopic labelling, 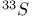 38 38
Isotopic substitution, 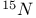 , , 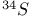 , , 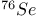 and and 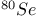 46 82 46 82
Jahn — Teller distortion 6 63 64 153 244 249—251
Macrocycles 287
Magnetic behaviour 7 68 219
Mass spectrometry 47
Matrix isolation 81 141
MCD spectroscopy 45
Metallic behaviour 218
Methane disulfonic acid, imido analogue 199
Methylenetriimido sulfate, ![$[CH_2S(N^tBu)_3]^{2-}$](/math_tex/650bfceb2b3881cce6d5f324cf27c51482.gif) 198 198
Microwave spectroscopy 32 141 144 146 168
Molecular conductors 68 217 226
Molecular wires 57 285
N,N'-Dimethylthionitrosamine, 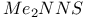 181 181
N,N'-Ureatotellurium imide 194
N-Halosulfinylamines 168
N-Thiosulfinylamines, RNSS 183
N-Thiosulfinylanilines 184
Negative hyperconjugation 48
Nitric oxide, biological storage 5
Nitric oxide, biological transport 171
Nitrogen diselenide, 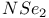 82 82
Nitrogen disulfide, 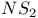 82 82
NMR spectroscopy, 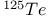 35 35
NMR spectroscopy, 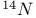 33 33
NMR spectroscopy, 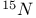 33—35 74 101 168 174 261 33—35 74 101 168 174 261
NMR spectroscopy, 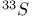 35 35
NMR spectroscopy, 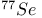 35 36 305 35 36 305
NMR, inverse detection 35
Norbornadiene adduct, 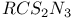 69 243 69 243
Norbornadiene adduct, 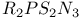 261 261
Norbornadiene adduct, 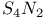 84 84
Norbornadiene adduct, ![$[S_3N_3]^+$](/math_tex/0391c895503cc8b2eea144e466624f1e82.gif) 95 95
Organoselenenyl azides, 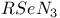 201 201
Organoselenium(IV) azides 202
Organosulfenyl azides,  201 201
Organotellurium(IV) azides 202
Organotin derivative, 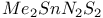 265 265
Oxidation states 2 8—10 296
Oxygen sensors 289
p-type doping 218 280
Paramagnetic ligand 220
Peierls instability 217
Pentaazidotellurite anion, ![$[Te(N_3)_5]^-$](/math_tex/57b94dcc9b51ac3db25e5bd78f55811682.gif) 90 90
Pentafluorosulfur amine,  145 145
Pentasulfur hexanitride, 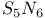 89 89
Perthionitrite anion, ![$[SSNO]^-$](/math_tex/6ea53a824e79f2b141758baef901bebd82.gif) 164 164
Phosphazene-sulfanuric rings, _y$](/math_tex/fcc083197220abc7b235e3bfd48788db82.gif) 270 270
Phosphazene-thiazyl rings,  268 268
Photoelectron spectroscopy 43
Photoisomerization 251
Poly(sulfur nitride), 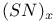 23 278—280 23 278—280
Poly(sulfur nitride), 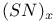 , bonding 56 57 279 , bonding 56 57 279
Poly(thionylphosphazenes) 286—289
Poly(thiophosphazene) 285
Polymer, $(SNBr_{0.4})_x 262
Polymers, 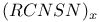 212 281 212 281
Radical dimerization 66—68
Raman spectroscopy 46
Reactivity patterns 47—48
Regiochemistry 69
Rietveld analysis, 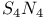 86 86
Ring contraction 74 87 153 174
Ring expansion 221 268
Ring-opening polymerization 269 285—287
S-Nitrososelenols, RSeNO 172
S-Nitrosothiols, RSNO 172
Sandwich complex,  115 115
Selenazyl halides, complexes 144
Selenazyl monomer, SeN 81
Seleninylamine 169
Selenium(IV) diimides, RNSeNR 185 187 193
Selenodiselenazyl dichloride, ![$[Se_3N_2Cl]Cl$](/math_tex/357c79b4da18974cf4f05bc96690490e82.gif) 149 149
Selenonitrosoarenes 182
Selenonitrosyl, metal complex 81 124
Selenonium azides, ![$[R_3Se]N_3$](/math_tex/da8e2b81223b1e8cd436145872df76d582.gif) 202 202
Silicon-nitrogen reagents 25
Spirocyclic sulfur(VI) system 269
Sulfanuric chloride, ![$[NS(O)Cl]_3$](/math_tex/7fba99d9afb71991eef8fa12aa70b38a82.gif) 24 153 24 153
Sulfanuric fluoride, ![$[NS(O)F]_3$](/math_tex/f6be280ee6e2e15d67d8101c8ba69a7d82.gif) 153 153
Sulfanuric polymers 269
Sulfanuric-phosphazene polymers see “Polythionylphosphazenes”
Sulfenamido anion, ![$[RSNR']^-$](/math_tex/a50149b85acc0d03658c9b36f957553882.gif) 203 203
Sulfenyl nitrenes, RSN 201
Sulfimide anion, ![$[NSPh_2]^-$](/math_tex/1d7b58d6d80fa10b2ff63fb0e713f31182.gif) , complexes 209 , complexes 209
Sulfinimidamide 191
Sulfinimidinates 170 191
Sulfonyl diimide, 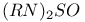 197 197
Sulfonyl imide, 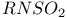 197 197
Sulfur diamides see “Diaminosulfanes”
Sulfur diimide anions, ![$[RNSN]^-$](/math_tex/4594df44c87588945dd0f2f18dd485f482.gif) 99 186 99 186
Sulfur diimide anions, ![$[RNSN]^-$](/math_tex/4594df44c87588945dd0f2f18dd485f482.gif) , complexes 135 , complexes 135
Sulfur diimide, HNSNH 99
Sulfur diimides, radical anions 190
Sulfur diimides, RNSNH 187
Sulfur diimides, RNSNR 186—193 281
Sulfur diimides, RNSNR, complexes 188
Sulfur diimides, unsymmetrical, ArNSNSAr' 284
Sulfur imide, SNH, complexes 131
Sulfur in liquid ammonia 34 101
Sulfur triimides,  146 197 146 197
Sulfur-nitrogen anions 34 98—103
Sulfur-nitrogen bonds, bent 113
Sulfur-nitrogen bonds, dissociation energies 172
Sulfur-nitrogen bonds, rotation barrier 172
Sulfur-nitrogen chains 56 57 281—285
Sulfur-nitrogen compounds, bonding 32
Sulfur-nitrogen radicals 37
Superconductor, 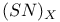 56 280 56 280
Syndiotactic 290
Tellurenamides 200
Tellurinylamines see “Imidotelluroxanes”
Tellurium azides 90
Tellurium nitride 89
Tellurium(II) diamide see “Diaminotellanes”
Tellurium(II) diamide, radical cation 201
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 ring
ring 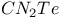 ring
ring 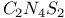 rings
rings 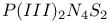 ring
ring 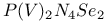 ring
ring 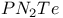 ring
ring ![$[Et_4P_2N_4S_2]^{2+}$](/math_tex/bf8b70dd65d241255cf0b30638718a6882.gif) dication
dication ![$[Ph_4P_2N_4S_2]^{2-}$](/math_tex/024b8b850e6208ea87906624e67dc99582.gif) dianion
dianion ![$[Te_2S_2N_4]^{2+}$](/math_tex/62da27831db6c6c9264594f513dc304e82.gif) dication
dication 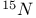 -enriched
-enriched 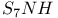
![$[S_3N]^-$](/math_tex/43084759b8c808f05b736141d660cbdb82.gif)
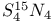
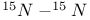 coupling
coupling 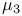 -Nitrido
-Nitrido 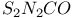
![$[N(SCl)_2]^+$](/math_tex/42962e7fd0fc3863d18923c3ece148ed82.gif)
![$[N(SeCl)_2]^$](/math_tex/c7977ff62ebeb7989e91d96d2e1fb1c382.gif)
![$[N(SeCl_2)_2]^+$](/math_tex/a70e4de54152688c93a149fddf433d1982.gif)
![$[NSCl_2]^-$](/math_tex/eee3c8fdab3daa78c8a7c62ddee8bf4382.gif)
![$[NSF_2]^-$](/math_tex/8e7ee00b2afdcd5c6f2897ea0e51a32382.gif)
![$[N_3Se_3O_6]^{3-}$](/math_tex/bcf484a2860b848d19aaacc9ac1faf4982.gif)
![$[N_3S_3O_3F_4]^-$](/math_tex/c8bae50383345587da7dce4b6822130582.gif)
![$[N_3S_3O_6]^{3-}$](/math_tex/c55f2a989216821f8d70ab43832f4ea682.gif)
![$[Se_2N_2]^{2-}$](/math_tex/4722f190e046fc6beb3c019a3ec5fe7482.gif) , complexes
, complexes ![$[Se_3N]^-$](/math_tex/f328b2547bcbe13cf15fb9c9452bde7c82.gif) , complexes
, complexes ![$[SNO]^-$](/math_tex/18cf5d81a268a47634a1e72312df83b782.gif)
![$[SN]^-$](/math_tex/5695f9f0e634df4e3f993965dfe7246082.gif) , complexes
, complexes ![$[SN_2]^{2-}$](/math_tex/2e96f88a782861b46d5636637485d98d82.gif)
![$[SO_2N_3]^-$](/math_tex/085606397b70df665422dbfa88eb4ef382.gif)
![$[SO_3N_3]^-$](/math_tex/2c4d4a56d241ae7357e218d7170e8c4382.gif)
![$[SSNO]^-$](/math_tex/6ea53a824e79f2b141758baef901bebd82.gif)
![$[SSNSS]^-$](/math_tex/a3688d9feecc97f9e108df8da42235ae82.gif)
![$[SSNS]^-$](/math_tex/bf8929925b1f768fa6d488aa136c123b82.gif)
![$[S_2N_2H]^-$](/math_tex/e3b0b676f2ec749b4da407f8f512c55982.gif)
![$[S_2N_2]^{2-}$](/math_tex/5a86942a3d778e334492d3e4d349a97c82.gif) , complexes
, complexes ![$[S_2N_3]^{3-}$](/math_tex/2d486d244b4a8b7ba541f99f1f14547582.gif) , complexes
, complexes ![$[S_3N_3O]^-$](/math_tex/da70cba52f9eebe36c01a823748ed50182.gif)
![$[S_3N_3O_2]^-$](/math_tex/df7dbb26a9bb08a10746151c8505f0c082.gif)
![$[S_3N_3O_4]^-$](/math_tex/754a9f83c08ca6a37a2938da5337f64582.gif)
![$[S_3N_3]^-$](/math_tex/5ed988a3ae98a787c6d2d605b6b9867682.gif)
![$[S_3N_4]^{2-}$](/math_tex/c11da2e47a1e6473ebf49ec016731c8782.gif) , complexes
, complexes ![$[S_4N_3]^-$](/math_tex/2c6b255e5ccc0d63a370006dcca3020a82.gif) , complexes
, complexes ![$[S_4N_4]^{2-}$](/math_tex/57e223b2246255f70d395b9f9f85284082.gif) , complexes
, complexes ![$[S_4N_5O]^-$](/math_tex/675a3a4f46e9c22b881eddeed075a97482.gif)
![$[S_4N_5O_2]^-$](/math_tex/11179ff73f528ddc9259a4548c6c612482.gif)
![$[S_4N_5]^-$](/math_tex/954f2c1d3cb46b072ffc9eec6efa94c582.gif)
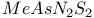
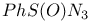
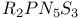
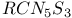
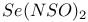
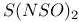
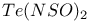
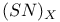
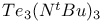
![$[S_5N_5]^+$](/math_tex/d4728a1e0b39682b9501cee433a9eec282.gif)
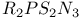
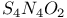
![$[S_4N_4]^{2+}$](/math_tex/b51e4240b524e660ef57c21590160d1f82.gif)

![$[S_3N_3]^+$](/math_tex/0391c895503cc8b2eea144e466624f1e82.gif)
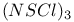
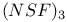

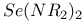
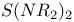
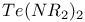
![$[(PhS)_2N]^{\bullet}$](/math_tex/5d647c987295f0ba036f125e575d2b2382.gif)
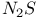
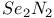
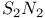
![$[RCTe_2N_2]^{\bullet}$](/math_tex/cf4d88039267db0fc500fa177733fa3e82.gif)
![$[S_2N]^+$](/math_tex/7cd31f1f66d6a6b29719c23597c84ba682.gif)
![$[S_2N_3]^+$](/math_tex/e0c5afd5eb7f943a5333245922a72e5582.gif)
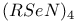
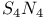
 )
) 
 interactions
interactions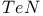 contacts
contacts  interactions
interactions 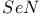 interaction
interaction 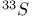
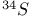 ,
, 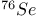 and
and 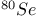
![$[CH_2S(N^tBu)_3]^{2-}$](/math_tex/650bfceb2b3881cce6d5f324cf27c51482.gif)
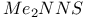
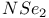
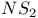
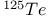
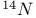
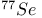
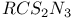
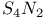
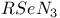

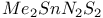
![$[Te(N_3)_5]^-$](/math_tex/57b94dcc9b51ac3db25e5bd78f55811682.gif)

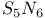
_y$](/math_tex/fcc083197220abc7b235e3bfd48788db82.gif)

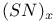
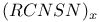

![$[Se_3N_2Cl]Cl$](/math_tex/357c79b4da18974cf4f05bc96690490e82.gif)
![$[R_3Se]N_3$](/math_tex/da8e2b81223b1e8cd436145872df76d582.gif)
![$[NS(O)Cl]_3$](/math_tex/7fba99d9afb71991eef8fa12aa70b38a82.gif)
![$[NS(O)F]_3$](/math_tex/f6be280ee6e2e15d67d8101c8ba69a7d82.gif)
![$[RSNR']^-$](/math_tex/a50149b85acc0d03658c9b36f957553882.gif)
![$[NSPh_2]^-$](/math_tex/1d7b58d6d80fa10b2ff63fb0e713f31182.gif) , complexes
, complexes 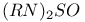
![$[RNSN]^-$](/math_tex/4594df44c87588945dd0f2f18dd485f482.gif)
