|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Flogge S. (ed.) — Encyclopedia of Physics. Thermodynamics of Gases |
|
|
 |
| Предметный указатель |
Heat conductivity coefficient of the monatomic gas 388
Heat conductivity coefficient of the monatomic gas mixture 410 423 451
Heat conductivity coefficient, 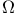 -integral representation 392 394 -integral representation 392 394
Heat conductivity coefficient, definition 301
Heat conductivity coefficient, higher approximations 401
Heat conductivity coefficient, measured values 395
Heat conductivity coefficient, pressure dependence 310
Heat conductivity coefficient, temperature dependence 400
Heat conductivity for ortho- and paramodifications 467
Heat conductivity, measurements 316 320
Heat flow 352 355
Heat flow vector 270
Heat flow, one-dimensional 290
Heat-conduction gauge 517 523 527
Helium isotopes, viscosity 463
Helium permeation through glass 634
Helium reemission from molybdenum 642
Helium, liquified 27
Helmholtz free energy 75
Hermite coefficients in terms of moment 269
Hermite expansion 267
Hermite expansion, absolute Maxwellian 267 280 284
Hermite expansion, locally Maxwellian 267 268 276
Hermite polynomials, eigenfunctions 272 281 283
Hermite polynomials, inversion formula 269
Hermite polynomials, tensor form 269
Hertz relation for the evaporation rate 541
High pressures, measurement 29 30
Highly dilute gases, solution of the Boltzmann equation for 364
Hilbert class of solutions of the Boltzmann equation 257
Hilbert expansion 252 254 258
Hilbert expansion, boundary condition 261
Hilbert expansion, compatibility condition 255
Hilbert expansion, continuation 258 259
Hilbert uniqueness theorem 252 257
Hilbert’s linear integral equation 366
Hoeppler viscosimeter 315
Houston ionization gauge 625 626 646
Hydrodynamical approximation 296 297 384 402 415 493 497
Impact parameter 234 236
Imperfect gases 91 100
Indicator for a test gas 542 544
Inelastic collision, equations 469
Influx (from leaks, etc.) 630 631seq.
Intermolecular forces, (6; Exp) potential 341 397
Intermolecular forces, Lennard — Jones potential 92 151 341 396 400 428 438 440
Intermolecular forces, power law 236 396 427 438
Intermolecular forces, rigid spheres, starve 340 343 394 395 427 437
Internal partition function 100seq. 121—123
Internal temperature 497
Inverse collisions 235
Inversion point 22
Inversion symmetry 359
Inversion temperature 27
Ion pump 515 621 640bis. 646
Ionization see Specific ionization
Ionization gauge 517 534 539 601
Ionization gauge of Bayard and Alpert 612 615
Ionization gauge of Houston, von Houston 625 626 646
Ionization gauge, calibration 619 620
Ionization gauge, conventional 611—612
Ionization gauge, linearity 617 618
Ionization gauge, pumping action 620—623 638 640 646
Ionization gauge, pumping speed measurement 644 645
Ionization gauge, sensitivity 616 617 623
Irreducible integrals 135 148
Irreversibility 360
Iso-mixture, definition 462
Isochores of the inert gases 17
Isothermal coefficient of bulk compressibility 6
Isothermal Joule — Thomson coefficient 21 38 50
Isotope effects on the partition function 118 121
Isotopic thermodiffusion factor 436 447
Isotropic orientation of angular momenta of molecules 489 491
Jet formation in valves, thermodynamics 566
Joints for ultrahigh vacuum 654
Joule coefficient 22 36
Joule expansion 8
Joule — Thomson coefficients 21 23 36 37 38 40 50
Joule — Thomson expansion 9
Joule’s law 7
Keyes and Beattie apparatus for compressibility measurements, Keyes — Beattlesche 31
Kihara approximation 392 401 436
Kinematical viscosity 300
Kinetic temperature 297
Kirkwood closure 157 159
Kirkwood’s derivation of the Boltzmann equation 230
Knife-edge vacuum seal 654 65 5
Knudsen formula for pressure force 531
Knudsen gas 212 214 216 245 288
Knudsen gas equation 241
Knudsen radiometer-vacuummeter 533
Knudsen’s laws of gas flow 617
Langmuir — Dushman molecular gauge 529 530
Laval valve 567 568 573 575—576 586
Law of corresponding states 48 51 62 98
Leak detection 541 542 661
Leak measurement 542 544
Lermard — Jones potential 92 151 341 396 400 428 438 440
Linde cycle 26 27
Linearity of an ionization gauge 617 618
Liouville theorem 84 85 470
Liouville theorem for collision 335 336
Liouville’s equation 208 252
Liquefaction of gases 26 28
Liquid helium pump 657
Liquid helium trap 657
Local entropy production 414
London’s law 339
Lorentz gas mixture 416
Loschmidt method 320
Mach angle 570
Mach number 567 569
Macrocanonical ensemble 74 79
Magnetic measurement of pressure 533
Manometer 655 (see also Gauge and Vacuummeter)
mass density 208 238
Mass diffusion equation 303
Mass spectrometer for measuring ultrahigh vacuum 626 627 635 643
Massey — Mohr formulae 459
Master equation 232
Maxwell relaxation effect 272
Maxwell — Boltzmann distribution function 363
Maxwellian gas 370 378 390 391
Maxwellian gas mixture 418 419
Maxwellian gas, distribution function 391
Maxwellian gas, eigentensors 390
Maxwellian gas, eigenvalues 377
Maxwellian molecules 237 270 284 344
Maxwellian transport equation 3 51
Maxwellian transport equation for a gas of rotating molecules 480 483
Maxwellian transport equationi experimental verification 363
Maxwellian velocity distribution 361
McLeod gauge 520 523
Mean free path 214
Mean mass velocity 303 355
Mean particle velocity 303 357
Mechanical pumps 547—559
Membrane gauge 517 519
Mercury manometer 519
Mercury vapour pump 547 559 591 646
Michel’s apparatus for compressibility measurements 32 33
Micro states, definitions 80
Microcanonical distribution 221
microcanonical ensemble 76 79
Mol-vacuummeter of Gaede 533
Molar fraction 303
Molar volume of gases 5
Molecular air pump 559 560 563
Molecular beam methods of vacuum investigation 540 541 603 604
| Molecular chaos 216 221 224 228 345 347
Molecular flow 515 530—531
Molecular force, (6; Exp) potential 341 397
Molecular force, elastic sphere 236
Molecular force, finite cut-off 211
Molecular force, Lennard — Jones potential 92 151 341 396 400 428 438 440
Molecular force, Maxwellian 237
Molecular force, power law 236 396 427 438
Molecular force, pseudo — Maxwellian 244
Molecular force, rigid spheres 340 343 394 395 427 437
Molecular gauge after Langmuir and Dushman 529 530
Molecular model, rough spheres 484 497 499
Molecular spin 301
Molecular thermal pressure 530 531
Molecular weight measurements 25 26
Moment equations for the 13 state variables 271 273 276
Motion reversibility 475
Multiple scattering of polarized electrons 481
Navier — Stokes equations 216 259 272 273 277 292
Normal deuterium, scattering cross section 463
Normal hydrogen, scattering cross section 462
Normal solutions of the Boltzmann equation 252 257 265
Nuclear spin contribution to the partition function 108 111
Number density 219
Oil air pump, rotary 516 547 549 553 559 593
Oil diffusion pump 594—605 647 649
Oil vapor, back diffusion 649
Oil vapor, thermal dissociation 649
Oil vapour pump 547 559 560 592 593
Onsager reciprocity law 283 407 410
Onsager — Casimir postulate 227 229
Ortho-hydrogen, partition function 110
Orthobaric density 43 45 53
Orthobaric volume 52 53
Orthohydrogen, scattering cross section 462
Pair distribution matrix 483
Para-hydrogen, partition function 110
Parahydrogen, scattering cross section 462
Partial pressure of a gas 58 64
Particle diffusion equation 303
Partition function 73 78 79 100
Penning vacuummeter 518 533 534
Perfect gas behaviour, deviation from it see Residual quantities
Perfect gas mixtures 57—59
Perfect gases 1 11
Perfect gases of Bose — Einstein type 174seq.
Perfect gases of Fermi’Dirac type 174seq. 179 182
Perfect gases, density matrix 192—196
Perfect gases, measurement of properties 28
Perfect gases, separation of degrees of freedom 85 91
Perfect gases, thermodynamic functions 5 8
Perfect-gas scale 2
Permeation of air through envelope of vacuum system 633seq.
Permeation rate of helium through glass 634
Phase function, definition 207
Phase space, definition 206
Phase transitions 96
Philips vacuummeter see Penning vacuummeter
Pirani resistance gauge 526
Piston gauge 29 30
Piston pump 547 548
Plait point of a binary mixture 65 66 67
Plane wave representation 185
Poincare recurrence theorem 219 226
Poisseuille formula 312
Polyatomic molecules, rotation and vibration levels 101 108
Potential of average force 156 157
Potential, chemical 73
Potential, chemical, of a gas mixture 61
Power law model 236 396 427 438
Poynting’s correction 70
Prandtl number, Prandtl — Zahl 301 394 333
Prandtl — Busemann characteristic line diagram 569 571
Pressure determination in vaccum, accuracy 518 519
Pressure determination in vaccum, magnetic method 533
Pressure diffusion 409
Pressure tensor 298 302 352
Pressure units (table) 516
Pressure values in mechanical pumps 556—558
Principle of corresponding states 48 51 62 98
probability density 153 160 218
Probability, conservation of 207
Pseudo-critical volume 49
Pseudo-Maxwellian molecule 244
Pumping action of ionization gauge 620—623 638 640 646
Pumping speed 629
Pumping speed of ionization gauge, measurement 644—645
Pumps, general choice of parameters 515
Pumps, Pumpen 544 605
Quantum mechanical effects in transport phenomena 452seq.
Quantummechanical parameter 453
Quartz-thread pendulum 527 528
Quasi — Lorentz-Gas 416
Quasi-stationary diffusion 320 321
Radiometer-vacuummeter 517 530 533
Real gas mixtures 59bis. 64
Rectilinear diameter see Cailletet — Mathias law
Reduced distribution function 208
Reduced equations 209 212
Reemission of helium from molybdenum 642
Reference states, definition 11
Relaxation and transport phenomena, relation 381 384
Relaxation time of a gas 368
Relaxation time of inner molecular motion 497 501
Residual energy 53 54 55
Residual free-energy 60
Residual gases in high vacuum 634 635
Residual heat capacity at constant volume 47 56
Residual pressure 12
Residual volume 13 16
Resistance gauge after Pirani 526
Retrograde condensation 66 67 68
Retrograde evaporation 66 67
Reversible expansion of a gas 8 27 28
Rigid sphere model 340 343 394 395 427 437
Roots pump 515 553 555 559
Rotary piston pump 552 553
Rotation levels of diatomic and polyatomic molecules 101 108
Rotational partition functions for diatomic molecules 111 113 122
Rotational partition functions for polyatomic molecules 113 115 122
Rough sphere model 484 497 499
Sage and Lacey apparatus for compressibility measurements 32
Scattering amplitude matrix 472
Scattering cross section of particles in gases 457 461
Scattering cross section, effective 490
Scattering matrix, symmetry relation 476
Scattering of light near the critical point 47 48
Scattering of particles in gases, classical limit 460
Scattering of particles in gases, quantummechanical treatment 455—485
Scattering phases 456
Scattering, representation of the cross section for viscosity and diffusion 458 461
Schleiermacher’s method of a coaxial wire 316
Seals for ultrahigh vacuum 653 655
Second virial coefficients 19 21 29 35 50 54
Second virial coefficients of a binary mixture 60 63
Self diffusion 427 430 431
Self diffusion coefficient 310
Self diffusion coefficient, measured values 427
Self diffusion for a gas of rough spheres 498
Self diffusion, temperature dependence 429
Semiconductors, use in resistance vacuum- meters 526
Senftleben effect 484 490
Sensitivity of an ionization gauge 616—617 623
Separation of degrees of freedom 85 91
Series developments for imperfect gases 123seq. 131seq. 134seq. 146 152
Shade theorem 473 480 487 490 491
Shear flow, unsteady 291
Shear viscosity for a gas of rough spheres 498
Shear viscosity for polyatomic gases 496
Shock 570 576
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -integral representation
-integral representation